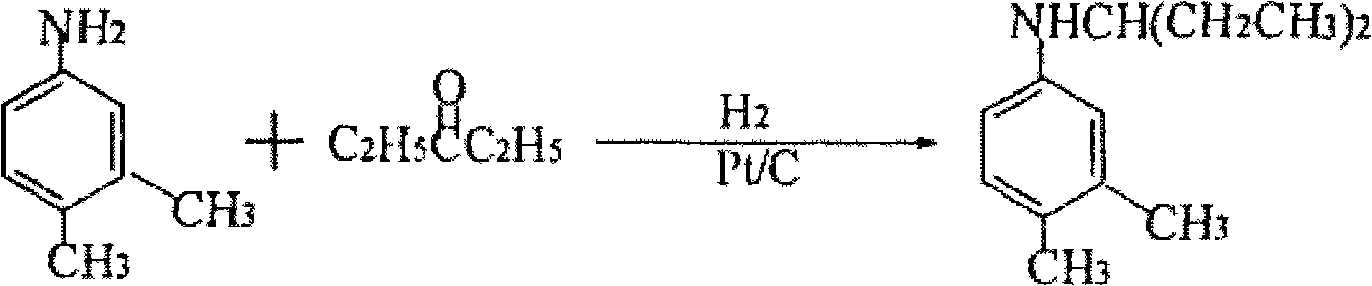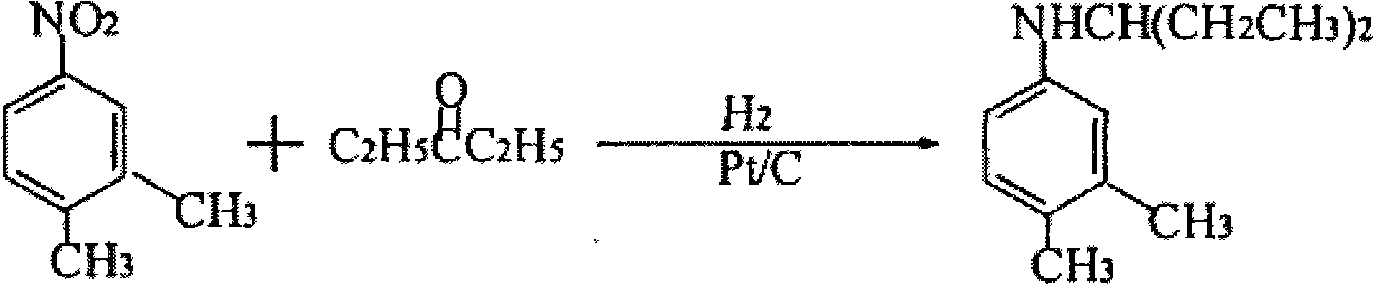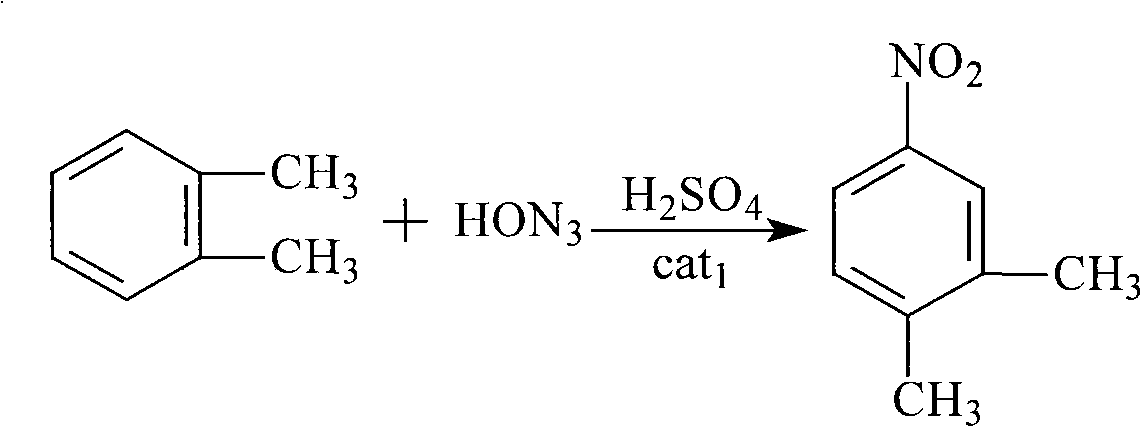Preparation of N-(1- ethyl propyl)-3,4-methyl toluidine
A technology of dimethylaniline and ethyl propyl, which is applied in the field of pendimethalin technical intermediates, can solve the problems of large amount of catalyst, easy decomposition and impact of 2-naphthalenesulfonic acid, etc., and achieve the goal of reducing by-products Formation, raw materials are cheap and easy to obtain, and the effect of simplifying the operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Nitration: Put 34.8Kg of concentrated sulfuric acid (98%, 348mol) into a 50L mixed acid configuration kettle, stir and slowly put 10.8Kg of concentrated nitric acid (98%, 168mol); in a 100L reactor, first add 12.84 Kg o-xylene (99%, 120mol), then add catalyst, slowly add mixed acid at 20-25°C, after the addition of mixed acid is completed, reflux reaction for 4-6 hours, and the reaction end point is controlled by HPLC. After passing the test, 13.65Kg of 3,4-dimethylnitrobenzene was obtained through rectification and separation, with a content of 99%.
[0033] (2) Hydrogenation, addition, hydrogenation: In a 100L pressure reactor, add 12.65Kg 3,4-dimethylnitrobenzene, 13.73Kg 3-pentanone (99%, 158mol), 0.05Kg Pd / C catalyst , control the pressure and feed hydrogen, heat up, start stirring, react until no hydrogen is absorbed, cool down, press out the kettle material, filter out the catalyst, separate the filtrate, distill the organic layer under reduced pressure, and ...
Embodiment 2
[0036] (1) Nitration: Put 34.8Kg of concentrated sulfuric acid (98%, 348mol) into a 50L mixed acid configuration kettle, stir and slowly put 10.8Kg of concentrated nitric acid (98%, 168mol); in a 100L reactor, first add 12.84 Kg o-xylene (99%, 120mol), then add catalyst, slowly add mixed acid at 20-25°C, after the addition of mixed acid is completed, reflux reaction for 4-6 hours, and the reaction end point is controlled by HPLC. After passing the test, 13.5Kg of 3,4-dimethylnitrobenzene was obtained through distillation and separation, with a content of 99.2%.
[0037] (2) Hydrogenation, addition, and hydrogenation: In a 100L pressure reactor, add 12.65Kg 3,4-dimethylnitrobenzene, 13.73Kg 3-pentanone (99%, 158mol), 0.05Kg Pd / γ- Al2O3 catalyst, control the pressure to feed hydrogen, raise the temperature, start stirring, and react until no hydrogen is absorbed, and the end point is controlled by gas chromatography. After qualified, lower the temperature, press out the kettle ...
Embodiment 3
[0040] Repeat the same steps as described in Example 1, but add 0.05Kg of improved polystyrene-supported palladium catalyst in step (2) hydrogenation, addition, and hydrogenation reactions, and measure the reaction end point by gas chromatography to finally obtain 14.95Kg N-(1-ethylpropyl)-3,4-dimethylaniline, content 99.3%.
[0041] All the other are with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com