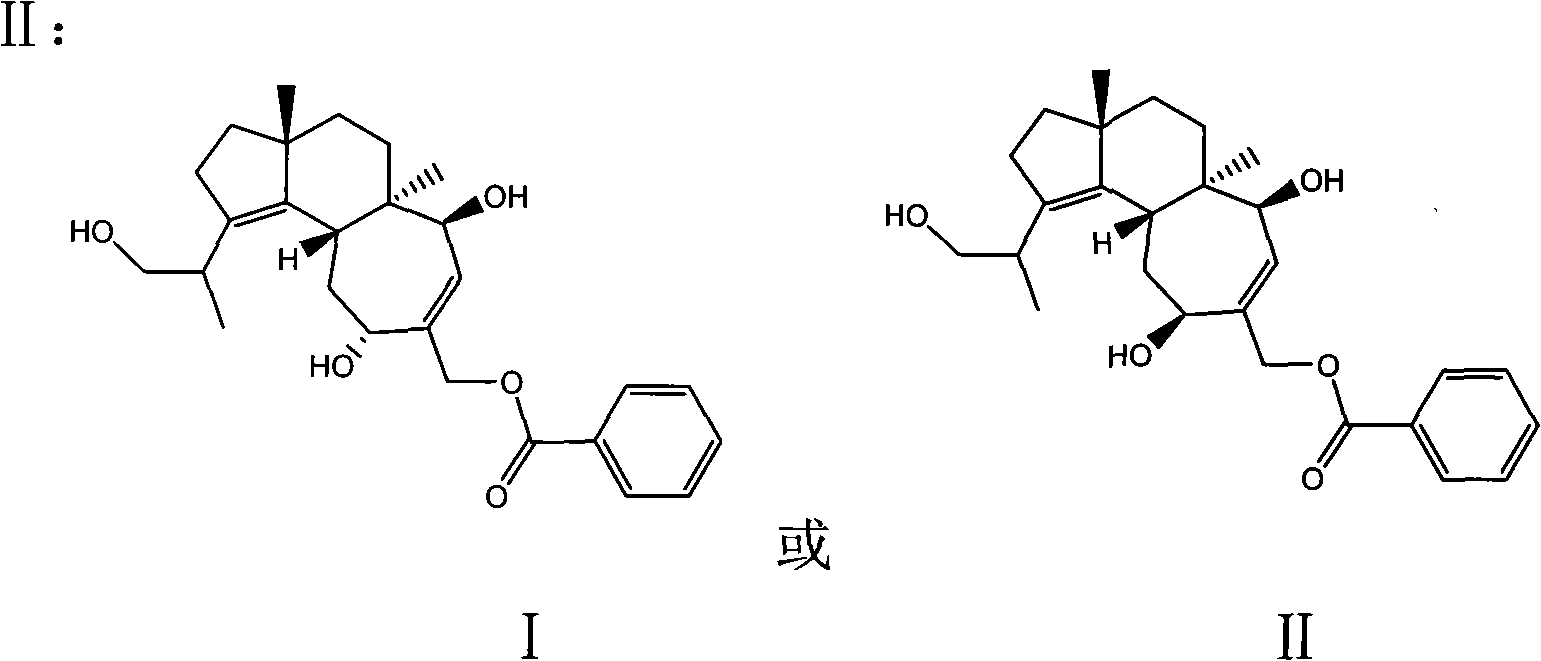
Nest alkane type diterpenoid and abstraction and separation method thereof
A separation method and compound technology, which is applied in the separation/purification of carboxylic acid esters, organic chemistry, medical raw materials derived from fungi, etc., can solve the problems such as no reports of bird's nest alkane-type diterpene compounds, and achieve cost reduction and Difficulty of operation, simple and feasible method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Soak 1 kg of dried fruiting bodies of C. crassa in methanol at room temperature for 36 hours, filter, recover methanol to obtain 65 g of extract. The extract was partitioned and extracted with chloroform-water, V (chloroform): V (water) = 6: 4, the solvent was concentrated and recovered to obtain 37 g of chloroform, and the aqueous phase was dissolved with methanol after concentration to obtain 22 g of methanol.
[0020] The chloroform part was transferred to a silica gel column, followed by V (petroleum ether): V (acetone) = 9: 1, V (petroleum ether): V (acetone) = 8: 2 eluent elution to remove impurities, and then use V (petroleum ether): V (acetone) = 7: 3 eluent eluted, collected eluent, recovered solvent.
[0021] The eluate obtained in the above steps was subjected to RP-8 column chromatography twice, and was eluted with the eluent of V (methanol): V (water) = 9: 1, and then through Sephadex LH-20, with 1: 1 Chloroform / methanol eluted and separated to obtain 10 m...
Embodiment 2
[0023] Soak 2kg of dry fruiting bodies of C. crassa in methanol at room temperature for 50 hours, filter, recover methanol to obtain 120g of extract. The extract was partitioned and extracted with chloroform-water, V (chloroform): V (water) = 5.5: 4.5, the solvent was concentrated and recovered to obtain 70 g of the chloroform portion, and the aqueous phase was dissolved with methanol to obtain 40 g of the methanol portion after concentration.
[0024] The chloroform part was transferred to a silica gel column, followed by V (petroleum ether): V (acetone) = 9: 1, V (petroleum ether): V (acetone) = 8: 2 eluent elution to remove impurities, and then use V (petroleum ether): V (acetone) = 7: 3 eluent eluted, collected eluent, recovered solvent.
[0025] The eluate obtained in the above steps was subjected to 3 times of RP-8 column chromatography, eluted with the eluent of V (methanol): V (water) = 8: 2, and then through Sephadex LH-20, with 1: 1 Chloroform / methanol eluted and se...
Embodiment 3
[0027] Soak 3kg of dried fruiting bodies of C. crassa in methanol at room temperature for 55 hours, filter, recover methanol to obtain 190g of extract. The extract was extracted with chloroform-water distribution, V (chloroform): V (water) = 5: 5, the solvent was concentrated and recovered to obtain 110 g of chloroform, and the aqueous phase was dissolved with hot methanol after concentration to obtain 68 g of methanol.
[0028] The chloroform part was transferred to a silica gel column, followed by V (petroleum ether): V (acetone) = 9: 1, V (petroleum ether): V (acetone) = 8: 2 eluent elution to remove impurities, and then use V (petroleum ether): V (acetone) = 7: 3 eluent eluted, collected eluent, recovered solvent.
[0029] The eluent obtained in the above steps was eluted with the eluent of V (methanol): V (water) = 7: 3 through 4 times of RP-8 column chromatography and RP-8 chromatography, and then subjected to Sephadex LH- 20, separated by elution with 1:1 chloroform / me...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com