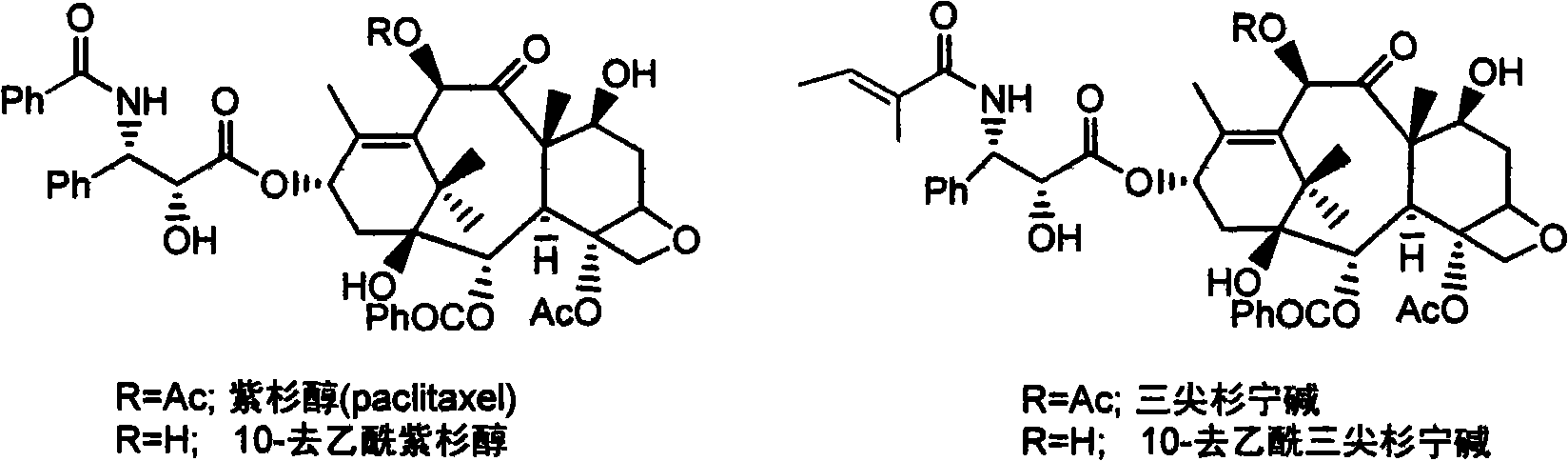
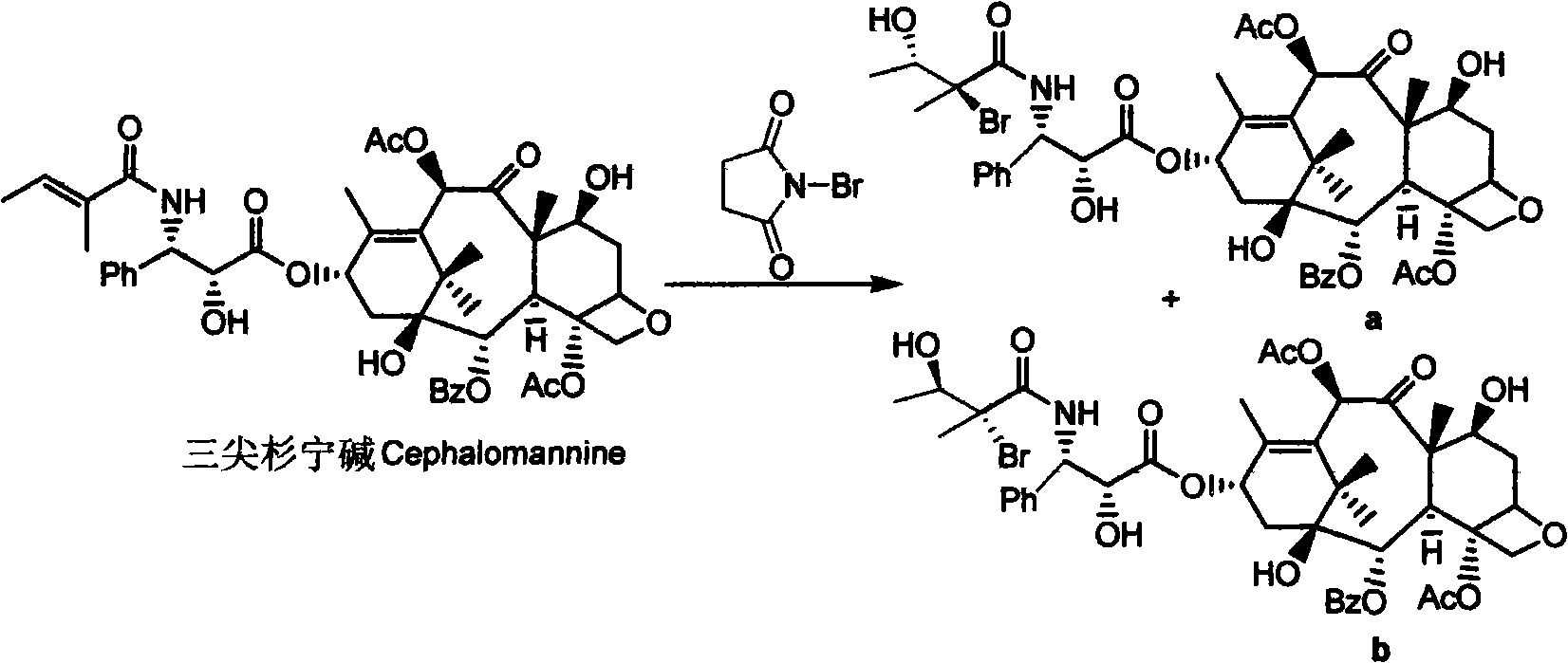
Method for separating and preparing paclitaxel
A technology of paclitaxel and taxane, which is applied in the field of preparation of paclitaxel raw materials, can solve the problems of unavailable price, strong carcinogenicity and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Weigh 1.0g taxane sample (containing 37% paclitaxel, 21% cephalomannine) and dissolve it in 20ml methanol, add 220mg NBS and Lewis acid, and react at room temperature. HPLC detection shows the conversion rate of cephalomannine After reaching 95%, the material liquid was evaporated to dryness under reduced pressure. After dissolving in about 8 ml of ethyl acetate-n-hexane (1:1), the sample is separated on the column, and eluted with ethyl acetate / n-hexane (1:1). Separately collect each component to obtain paclitaxel (purity>90%). The various Lewis acids used, their dosage, reaction time, and paclitaxel yield are listed as follows:
[0040] Table 1. The addition of monobromomonoalkoxy catalyzed by various Lewis acids
[0041]
Embodiment 2
[0043] Weigh 1.0g taxane sample (containing 37% paclitaxel, 21% cephalomannine) and dissolve it in 40ml methanol:water (3:1) system, add 220mg NBS, and react at 35℃ for 12 hours. HPLC detection shows The conversion rate of cephalomannine is 95%. After the addition reaction, the product was extracted three times (40ml, 10ml, 10ml) with ethyl acetate-n-hexane (1:1), and the extracted phases were combined and concentrated. The concentrated solution was dissolved in about 8 ml of ethyl acetate-n-hexane (1:1), and then separated on a sample column, and eluted with ethyl acetate / n-hexane (5:5). Separately collect each component to obtain 378 mg of paclitaxel with a purity of 91%. The yield was 93%.
Embodiment 3
[0045] Weigh 5.00g taxane sample (37% paclitaxel, 21% cephalomannine) and dissolve it in 100ml methanol, add 10ml water and 1g NBS, react at 35℃ until cephalomannine disappears, and use ethyl acetate as the product Ester-n-hexane (1:1) was extracted three times (80ml, 40ml, 20ml), combined and concentrated. The concentrated solution was dissolved in 40 ml of ethyl acetate-n-hexane (1:1) and separated by column. Elute with ethyl acetate / n-hexane (5:5)-ethyl acetate / n-hexane (4:6). After separate collection, 1.65 g of paclitaxel was obtained, with a purity of 90%. The yield was 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com