Process for the preparation of thermoplastic polyurethanes based on 1,5-naphthalene-diisocyanate
A technology of thermoplastic polyurethane and diisocyanate, which is applied in the field of preparation of thermoplastic polyurethane based on 1,5-naphthalene-diisocyanate, and can solve problems such as prepolymer instability and side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1: Preparation of NDI-based, storage-stable NCO prepolymers (according to the invention)
[0077] Dehydrate 100 parts by weight (pbw) of poly-e-caprolactone derived from neopentyl glycol and having a hydroxyl number of 70 mg KOH / g, at 118° C. with 26.03 pbw of Desmodur 15 polyisocyanates were stirred together. After 11 minutes, the reaction temperature rose to 129°C. The mixture was cooled to 65°C over 10 minutes. The prepolymer was divided into samples which were analyzed after different storage times to determine viscosity (measured at 120°C), appearance (evaluated at a temperature of 50°C) and NCO values (see Table 1).
[0078] Table 1 : Storage conditions and properties of prepolymers
[0079] Prepolymer No.
[0080] The storage conditions selected in Table 1 cover every conceivable temperature exposure the prepolymers may be subjected to after preparation. So, for example, "16 h at 65°C" (prepolymer 1.1, Table 1) simulates a cooling o...
Embodiment 2
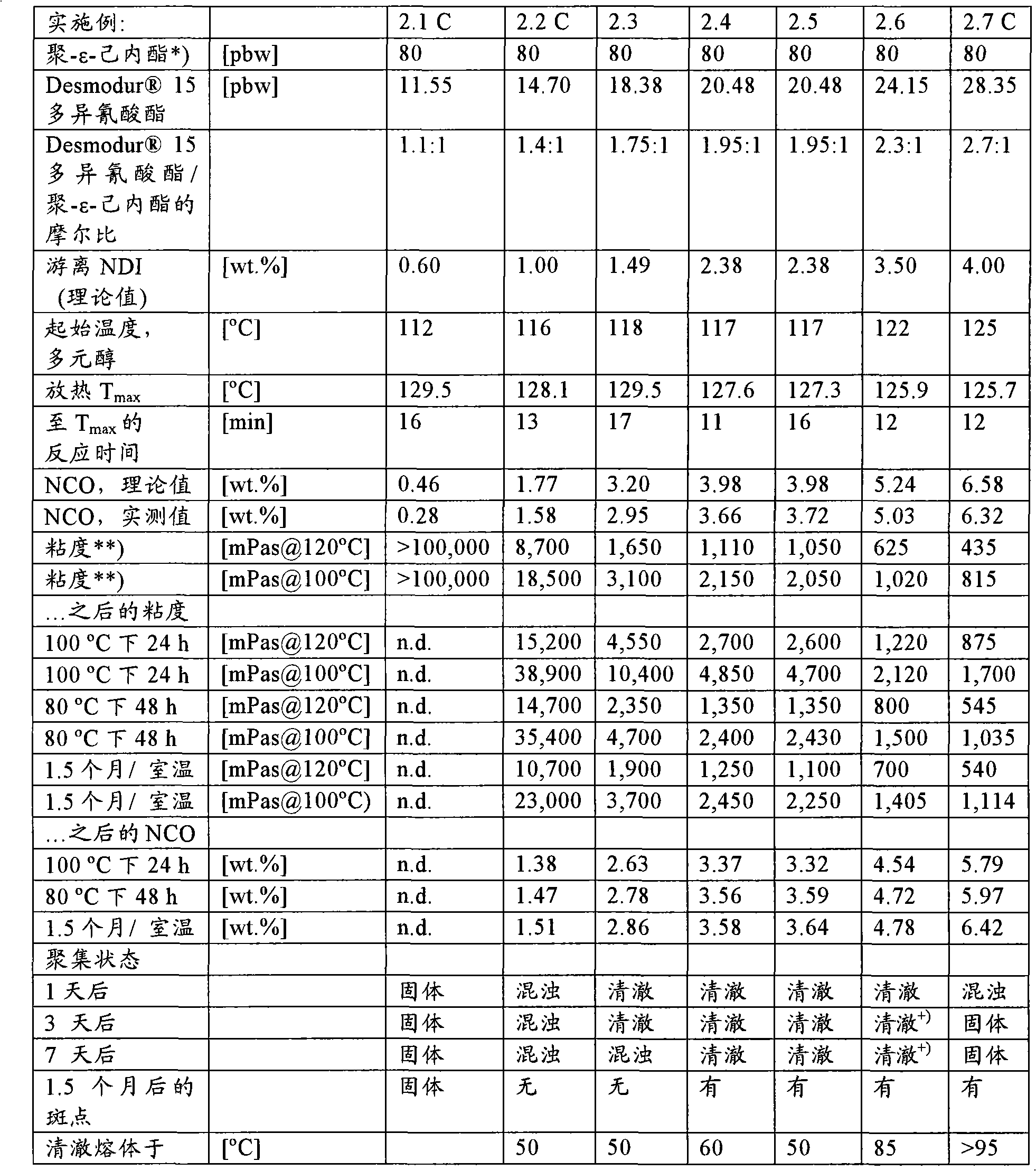
[0081] Example 2: Preparation of NCO prepolymers used and not used according to the invention
[0082] Prepolymers were prepared as described in Example 1. The formulation and properties of the prepolymer are shown in Table 2.
[0083] For example, prepolymer 2.4 (Table 2) was prepared as follows:
[0084] Dehydration of 80 pbw poly-ε-caprolactone originating from neopentyl glycol and having a hydroxyl number of 70 mg KOH / g, at 118° C. with 20.48 pbw of Desmodur 15 polyisocyanates were stirred together. After 11 minutes, the reaction temperature rose to 129°C. The mixture was cooled to 65°C over 10 minutes. The prepolymer was divided into several samples, and the samples were analyzed after different storage times (24h, 48h and 1.5 months) at different storage temperatures (room temperature, 80°C and 100°C), and the viscosity (at 100 and 120°C measurement), appearance (evaluated at a temperature of 23°C) and NCO values (see Table 2).
[0085] Table 2 : Preparati...
Embodiment 3
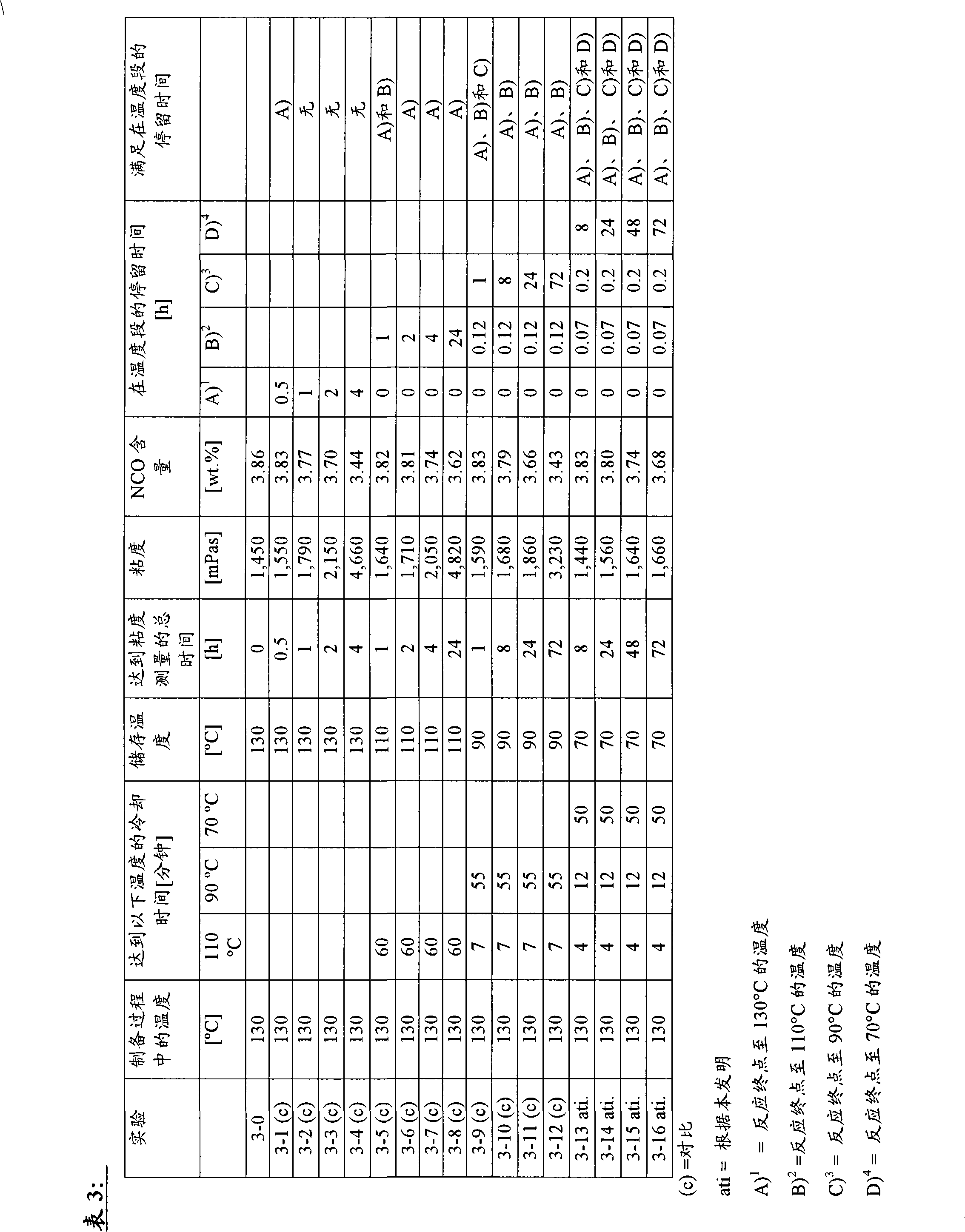
[0093] Analogously to Example 2, 100 pbw of poly-ε-caprolactone originating from neopentyl glycol and having a hydroxyl number of 70 mg KOH / g were dehydrated at 118° C. with 26.03 pbw of Desmodur 15 polyisocyanates were stirred together. After 11 minutes, the reaction temperature rose to 130°C (reaction end point). Thereafter, the mixture is divided into different batches, which are subjected to different temperatures and storage times. Due to the relatively small amount, the time required to reach the specified storage temperature varies and is in the range of minutes. In order to establish the comparability of the measurement results, the viscosity was recorded at 100°C using a "Physica MCR 51" viscometer from Anton Paar regardless of the previously determined storage temperature. The NCO content was measured in each case by methods known to those skilled in the art, ie reaction with excess dibutylamine and back titration thereof.
[0094]
[0095] Table 3 shows that ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com