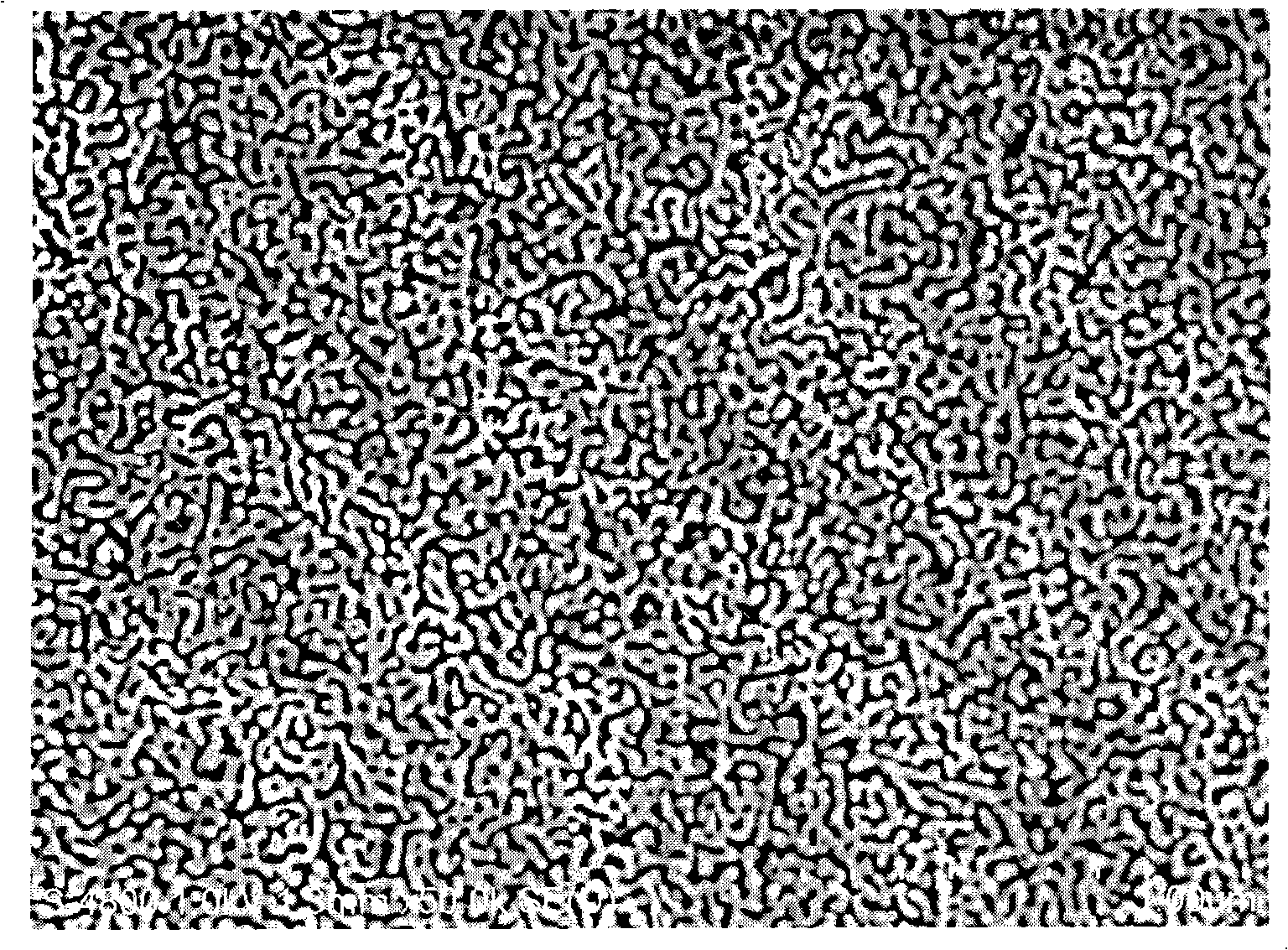Method for coating noble metal on nano porous gold and prepared catalyst
A nanoporous gold, nanoporous technology, applied in metal/metal oxide/metal hydroxide catalysts, catalyst activation/preparation, physical/chemical process catalysts, etc., can solve the problem of large deposition of precious metals, and achieve high chemical resistance. Electrochemical corrosion, good electron transport properties, wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A commercial gold-silver alloy foil with a length of 2cm, a width of 1.8cm, and a thickness of 100nm was transferred to 68wt.% concentrated nitric acid and corroded for 30 minutes at room temperature (28°C) to prepare a nanoporous gold film. The SEM photo is as follows: figure 1 shown.
[0037] The prepared nanoporous gold film was immobilized on a glassy carbon (GC) electrode at 0.5 mmol dm -3 Soak in the chloroplatinic acid solution for 30 seconds, and some chloroplatinic acid ions are adsorbed on the nanoporous gold surface.
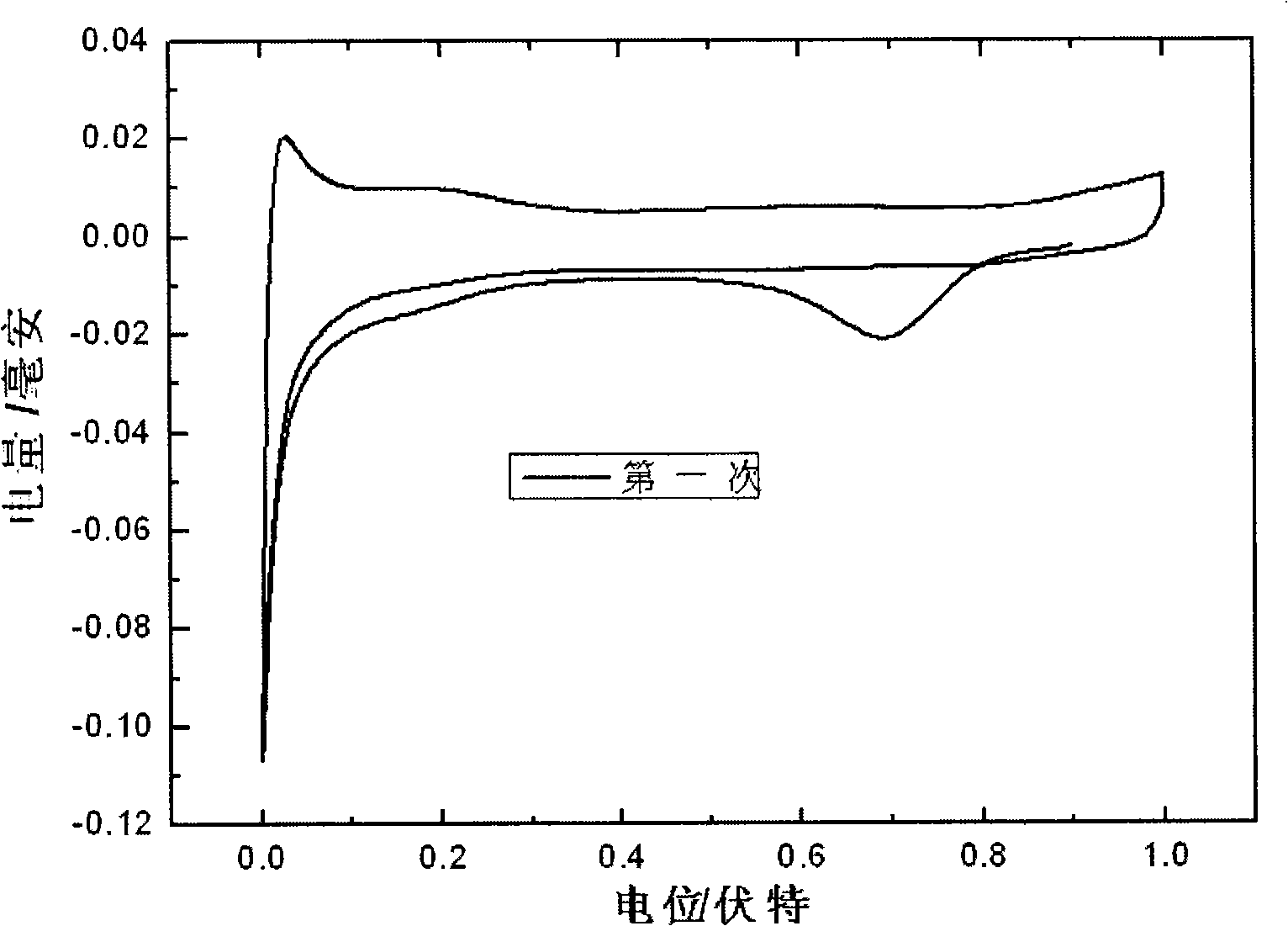
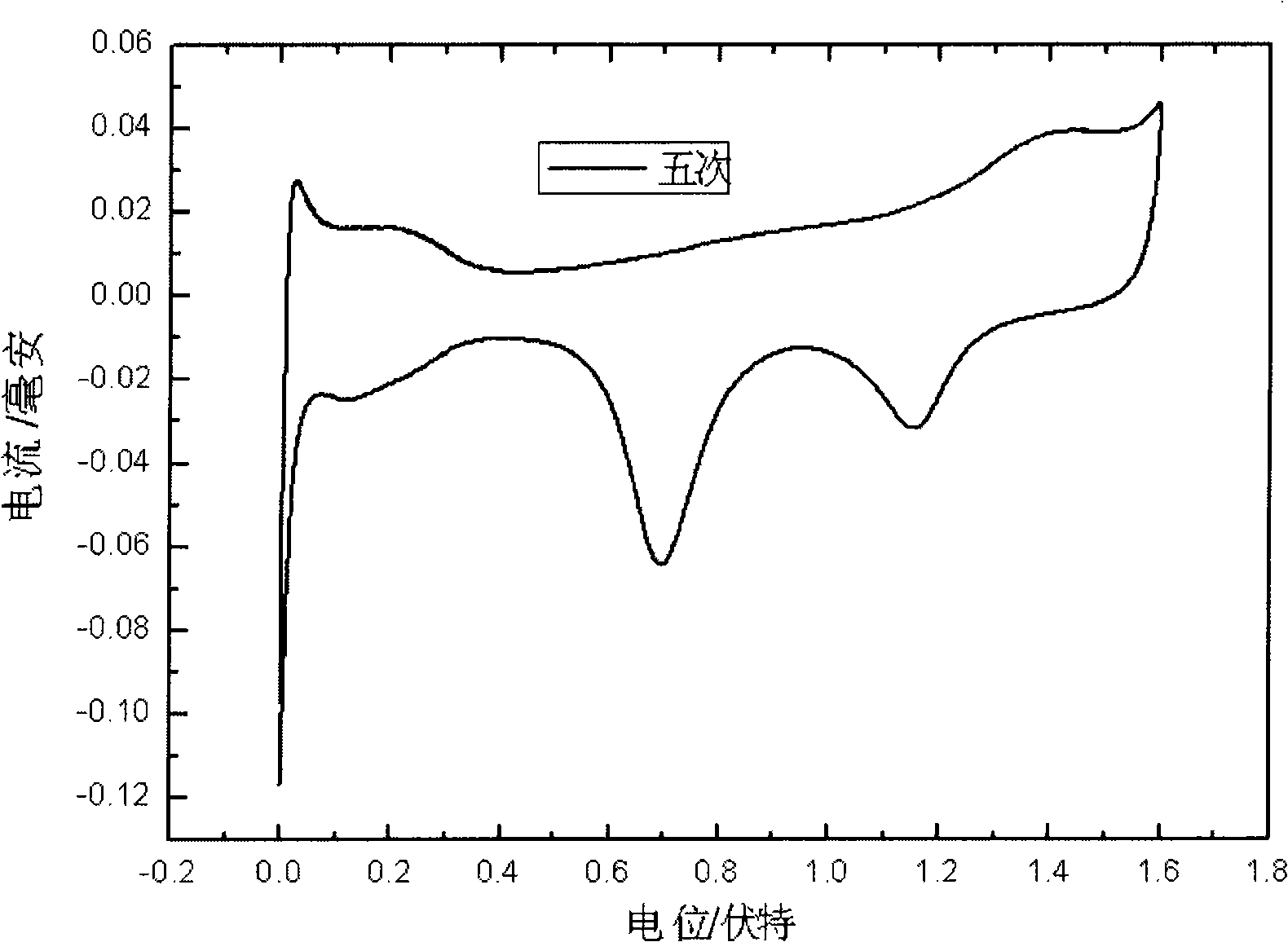
[0038] The nanoporous gold electrode adsorbed with chloroplatinate ions was washed three times with deionized water, each time for 5 minutes, and then under the three-electrode system, using reversible hydrogen as a reference, the chlorine adsorbed on the nanoporous gold surface was reduced by potential linear scanning Platinate ion. The reduction charge of chloroplatinate ion is as figure 2 As shown, the amount of modified platinum can be ...
Embodiment 2
[0041] A commercial gold-silver alloy with a length of 2 cm, a width of 1 cm, and a thickness of 25 μm was transferred to 68 wt.% concentrated nitric acid and corroded for 1 h at room temperature (28° C.) to prepare thick nanoporous gold.
[0042] Prepared nanoporous gold in 1mmol dm -3 Soak in the chloroplatinous acid solution for 10 minutes, and adsorb some chloroplatinous acid ions on the porous gold surface.
[0043] The nanoporous gold electrode adsorbed with chloroplatinous acid ion was washed three times with deionized water, 30 min each time, and then under the three-electrode system, with reversible hydrogen as a reference, the electrode adsorbed on the nanoporous gold surface was reduced by potential linear scanning. Platinum-modified nanoporous gold composite catalysts prepared from chloroplatinite ions.
Embodiment 3
[0045] (1) A gold-silver alloy with a thickness of 20 μm, a width of 3 cm, a length of 10 cm, and a gold mass ratio of 37% is placed in nitric acid with a concentration of 65 wt.%, at a temperature of 30° C., corroded for 20 min, and then deionized Wash the surface of the alloy and the nitric acid in the pores to obtain nanoporous gold;
[0046] (2) Fix the nanoporous gold on the glassy carbon electrode and place it at a concentration of 1mol dm -3 In the chloropalladium acid solution, soak 10min, allow the nanoporous metal surface to absorb chloropalladium acid ion;
[0047] (3) Place the nanoporous gold with chloropalladate ions adsorbed on the surface in a metal ion-free solution or deionized water, soak and wash for 10 minutes, replace with a new metal ion-free solution or deionized water, soak for another 15 minutes, repeat 5 times , until the nanoporous gold surface is cleaned;
[0048] (4) Place the nanoporous metal with clean surface adsorption of chloropalladate ion...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com