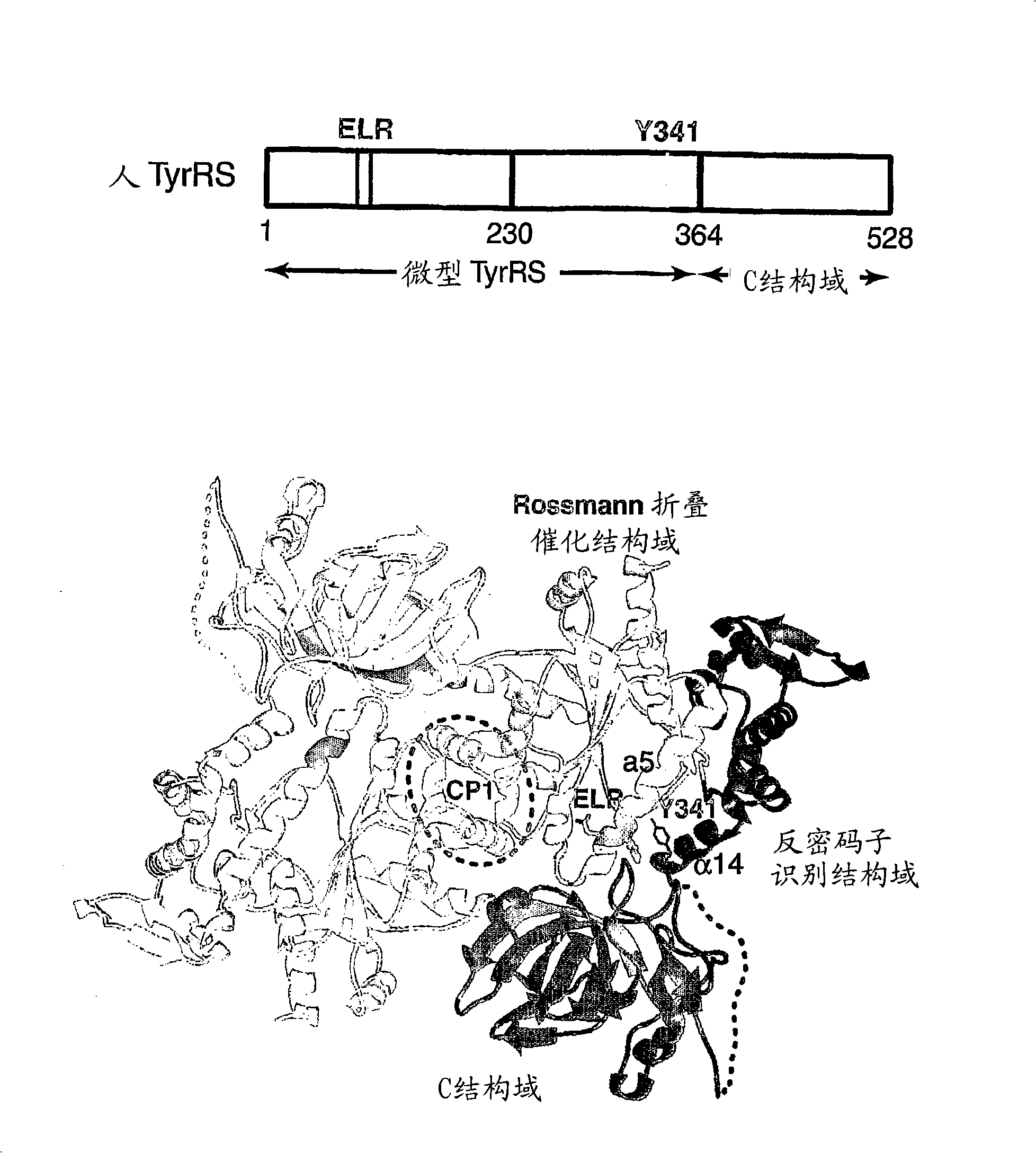
Angiogenic tyrosyl trna synthetase compositions and methods
An angiogenesis and enzyme synthesis technology, applied in the field of angiogenic TyrRS protein variants and its angiogenic fragments to stimulate angiogenesis, can solve the problem of no cytokine activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0026] DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT
[0027] Amino acid residues in proteins have been classified in different ways, mainly based on the physicochemical characteristics conferred by the amino acid side chains. For example, one general classification includes three classes of amino acids: (1) hydrophobic (nonpolar) amino acids, including glycine, alanine, valine, phenylalanine, proline, methionine, isoleucine, amino acids; (2) charged amino acids, including aspartic acid, glutamic acid, lysine, and arginine; and (3) polar amino acids, including serine, threonine, tyrosine, Histidine, cysteine, asparagine, glutamine, and tryptophan; glycine is sometimes included in the fourth class, by itself (see Chapter 1 of Branden and Tooze, Introduction to Protein Structure, Second Edition, Garland Publishing, Inc. 1998, pages 3-12, which are incorporated herein by reference).
[0028] Amino acid residues can be further classified according to whether their side chains...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com