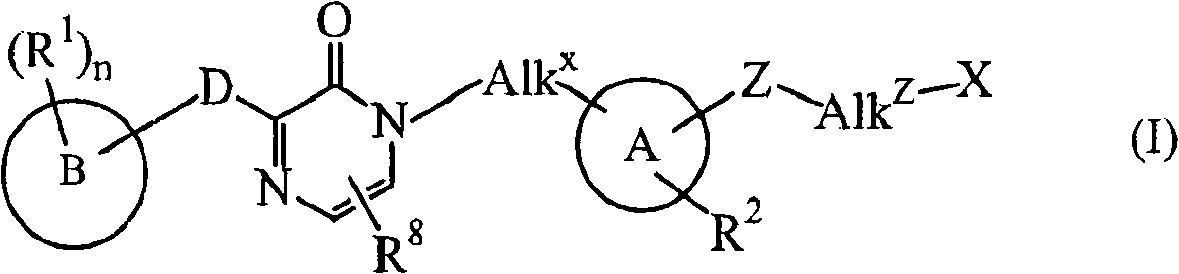
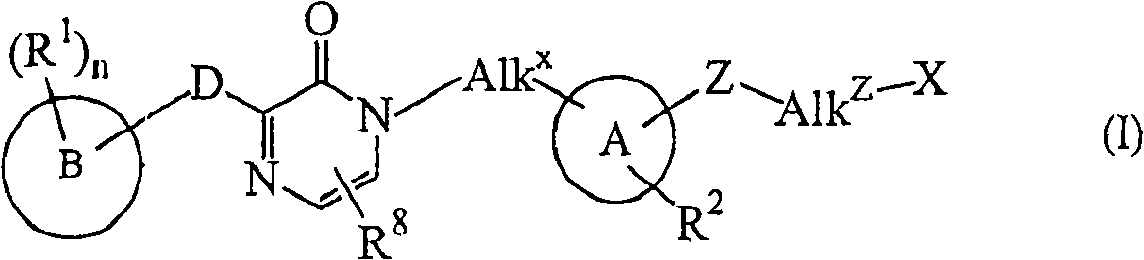
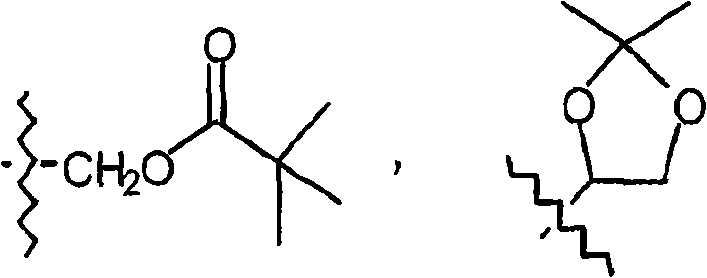Novel substituted pyrazinone derivatives for use in MCH-1 mediated diseases
A technology of pyrazinone and derivatives, applied in the field of pyrazinone derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A1
[0178] a) Preparation of intermediate compound 1
[0179]
[0180] React in the microwave. 2-phenoxyethylamine (0.010mol), 2,3 dichloropyrazine (0.012mol) and 1,8-diazabicyclo(5.4.0.)undec-7-ene (DBU)(0.012 mol) in CH 3 The mixture in CN (20ml) was heated at 180°C for 20 minutes. The solvent was evaporated. The residue was purified by an open silica gel short column (eluent: CH 2 Cl 2 ). The product fractions were collected and the solvent was evaporated. Obtained: 2.5 g of intermediate compound 1 (quantitative yield; used in the next reaction step without further purification).
[0181] b) Preparation of intermediate compound 2
[0182]
[0183] React in the microwave. A mixture of intermediate compound 1 (0.0092 mol) in NaOH (10 ml; 50%) and DMSO (10 ml) was heated at 150° C. for 30 minutes. The reaction mixture was cooled to 0 °C. EtOAc and water were added at 0 °C. The organic layer was separated and dried (Na 2 SO 4 ), filter Dicalite and evaporate...
Embodiment A2
[0185] Preparation of intermediate compound 3
[0186]
[0187] Stir 4-bromo-2-methoxyphenol (0.0246mol), 2-hydroxyethylpyrrolidine (0.0492mol) and triphenylphosphine (0.0492mol) in THF (50ml; anhydrous) at 0°C in the mixture. In a microwave oven, diethyl azodicarboxylate (0.0492 mol) was added at 0°C. The reaction mixture was stirred at 100°C for 5 minutes. Join Na 2 CO 3 aqueous solution. The mixture was extracted with EtOAc. The separated organic layer was dried (Na 2 SO 4 ), filtered and evaporated the solvent. The residue was captured by adding Amberlyst 15 (0.123 mol) followed by NH 3 / CH 3 OH to release it, followed by purification through an open silica gel short column (eluent: CH 2 CI 2 (CH 3 OH / NH 3 )95 / 5). The product fractions were collected and the solvent was evaporated. Obtained: 6.7 g of intermediate compound 3 (91%).
Embodiment A3
[0189] a) Preparation of intermediate compound 4
[0190]
[0191] A mixture of NaH (0.0049 mol; 60%) in DCE (1.7 ml) was stirred at 0°C. At 0°C, a solution of 2-phenylethanethiol (0.0031 mol) in DCE (5.6 ml) was added in portions. The reaction mixture was stirred at room temperature for 30 min. A solution of 2,3-dichloropyrazine (0.0033 mol) in DCE (1.7 ml) was added and the resulting reaction mixture was heated in a microwave oven at 80° C. for 10 minutes. The mixture was filtered through Celite and the residue on the filter was washed with CH 2 Cl 2 . The solvent of the filtrate was evaporated. The residue was purified by an open silica gel short column (eluent: CH 2 Cl 2 / Hexane 1 / 1, then CH 2 Cl 2 ). The product fractions were collected and the solvent was evaporated. Obtained: 0.5 g of intermediate compound 4 (72%).
[0192] b) Preparation of intermediate compound 5
[0193]
[0194] React in the microwave. A mixture of intermediate compound 4 (0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com