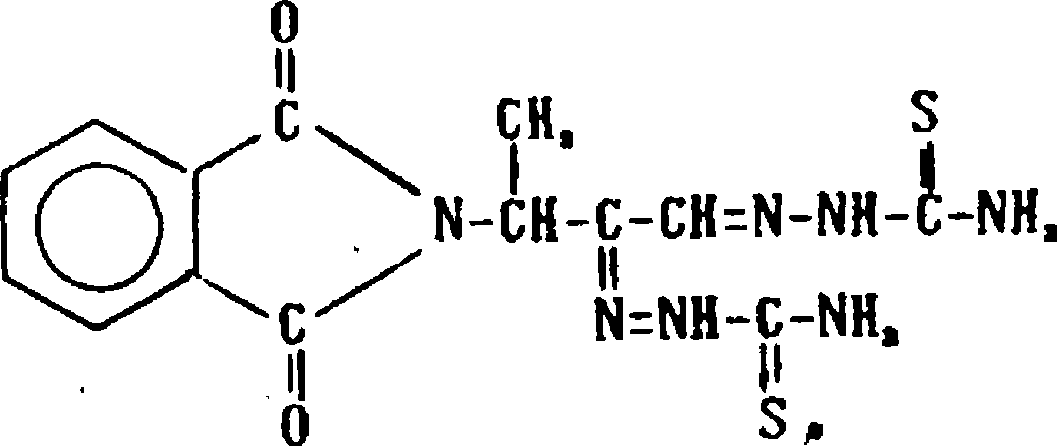Phthiobuzonum/diclothane compound topical formulation
A topical preparation technology, applied to the compound topical preparation of phthalidene/dyclonine and its preparation, in the field of treating herpes simplex and herpes zoster skin diseases, so as to improve the quality of life, prevent infection, and improve clinical compliance sexual effect
Active Publication Date: 2011-01-05
BEIJING HUMANWELL JUNWEI PHARM TECH CO LTD
View PDF1 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
At present, there is no report on the combination of phthalide and dyclonine and its use in the treatment of viral skin diseases such as herpes zoster
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
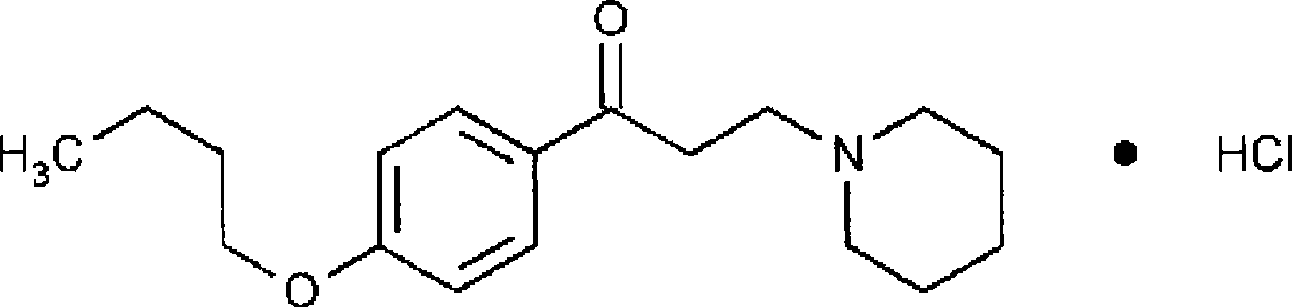
The invention discloses a compound topical preparation of fitbamzone dyclonine used for curing viral skin diseases, such as herpes zoster, etc. The topical preparation of fitbamzone dyclonine complex prescription consists of following components by percentage composition: (1) 0.1 to 10 weight percent of the fitbamzone, (2) 0.1 to 10 weight percent of the dyclonine, and (3) pharmaceutically acceptable excipient. The compound topical preparation used for curing the herpes zoster can stop pain, immediately relieve the suffering of a patient to the maximum, improve the life quality of the patientand contribute to improve the clinical compliance of the patient based on ensuring the curative effect of anti-virus and achieving the purpose of treatment. The compound topical preparation has the advantages of having an evident curative effect on the herpes zoster, a good analgesic effect, stable preparation performance, controllable quality, a good curative effect, and nonirritant to skin without causing allergic reaction.
Description
A compound topical preparation of phthalobutane / dyclonine technical field The invention relates to topical pharmaceutical preparations, more specifically, a compound topical preparation of phthalobutane / dyclonine and a preparation method thereof, which are used for treating herpes simplex and herpes zoster skin diseases. Background technique Virus infection has always been a serious social topic that people care about. Virus infection causes multiple diseases and seriously threatens people's health. Viral skin diseases occupy an important position in skin diseases, and herpes zoster (Herpes Zoster) is caused by varicella zoster. The acute inflammatory skin disease caused by herpes zoster virus is called "waist-wrapped fire dragon" and "waist-wrapped fire pill" in traditional Chinese medicine, commonly known as "spider sore". Its main features are clusters of blisters distributed in clusters along one side of the peripheral nerve, accompanied by obvious neuralgia. The firs...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): A61K9/107A61P17/00A61K9/06A61K31/472A61P31/12A61K9/00A61K31/435
Inventor 高永良李志红王晋李劲彤
Owner BEIJING HUMANWELL JUNWEI PHARM TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com