Biodegradable sustained-release medicament stent for TMR and preparation method
A degradable and degradable polymer technology, applied in stents, medical science, surgery, etc., can solve the problems of tumor-promoting mutagenesis, poor targeting, and less research on myocardial stents, and achieve the effect of promoting angiogenesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
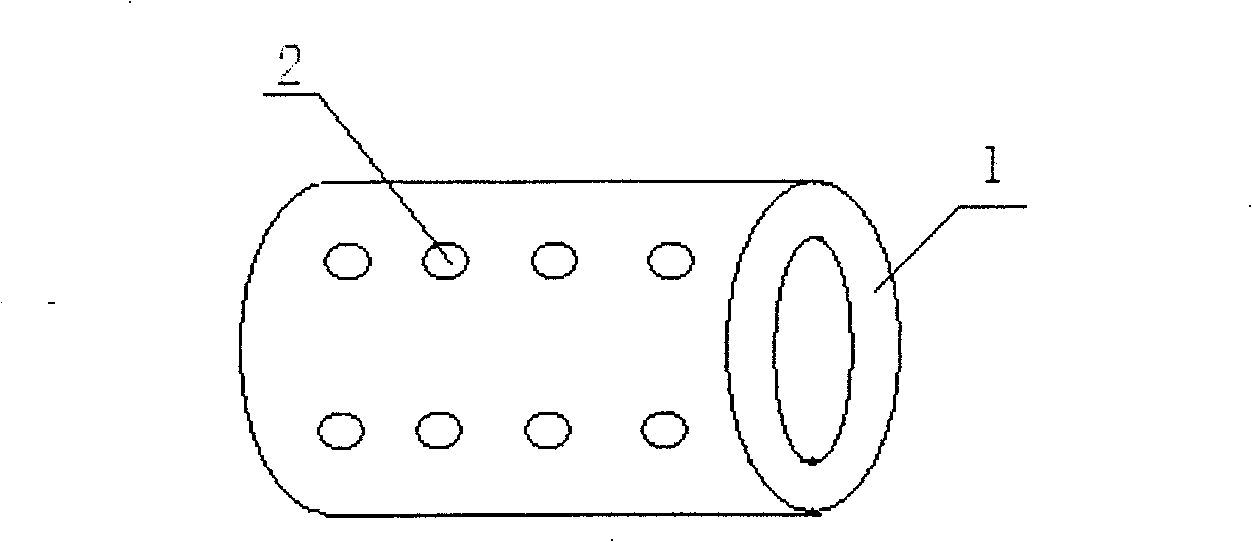
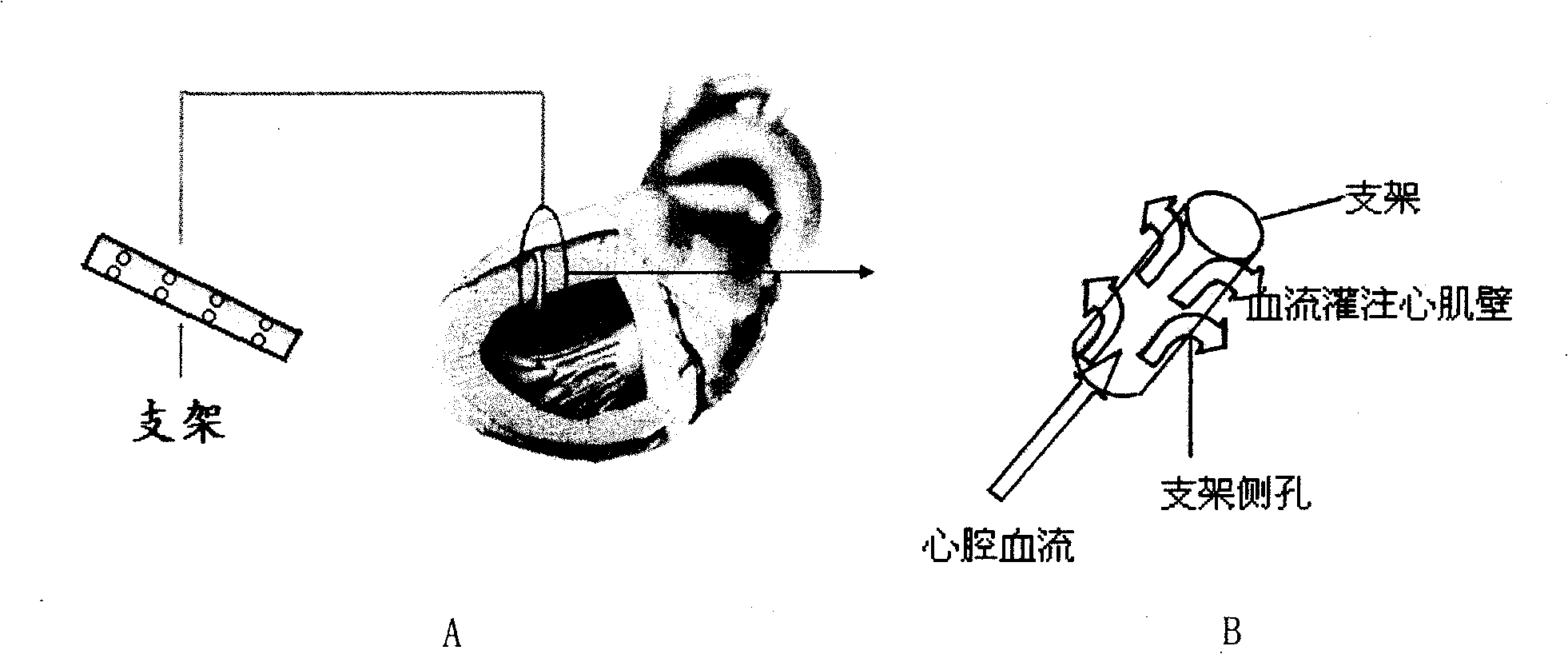
[0028] A biodegradable slow-release stent for transmural myocardial revascularization, comprising a hollow tube 1, the inner diameter of the tube is 0.5 mm, the wall thickness of the tube is 0.3 mm, and the length of the tube is 0.3 mm. mm, the wall of the tube is provided with a small hole 2 through the wall, the material of the tube is polycaprolactone loaded with basic fibroblast growth factor and heparin, the basic fibroblast growth factor, heparin and The ratio of polycaprolactone is 30ug:20mg:0.5g.
Embodiment 2
[0030] A biodegradable drug slow-release stent for transmural myocardial revascularization, comprising a hollow tube 1, the inner diameter of the tube is 0.2 mm, the wall thickness of the tube is 0.1 mm, and the length of the tube is 0.1 mm. mm, the wall of the tube is provided with a small hole 2 through the wall, the material of the tube is polylactic acid loaded with vascular endothelial growth factor and heparin, and the ratio of the vascular endothelial growth factor, heparin and polylactic acid is 50ug: 20mg:0.1g.
Embodiment 3
[0032] A biodegradable slow-release stent for transmural myocardial revascularization, comprising a hollow tube 1, the inner diameter of the tube is 1 mm, the wall thickness of the tube is 0.5 mm, and the length of the tube is 0.5 mm , the wall of the tube is provided with a small hole 2 through the wall, the material of the tube is polyglycolic acid loaded with β-type transforming growth factor and heparin, the β-type transforming growth factor, heparin and polyglycolic acid The ratio is 20ug:50mg:1g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com