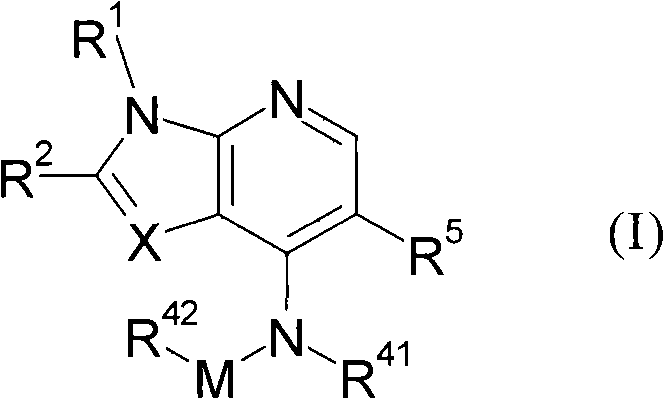
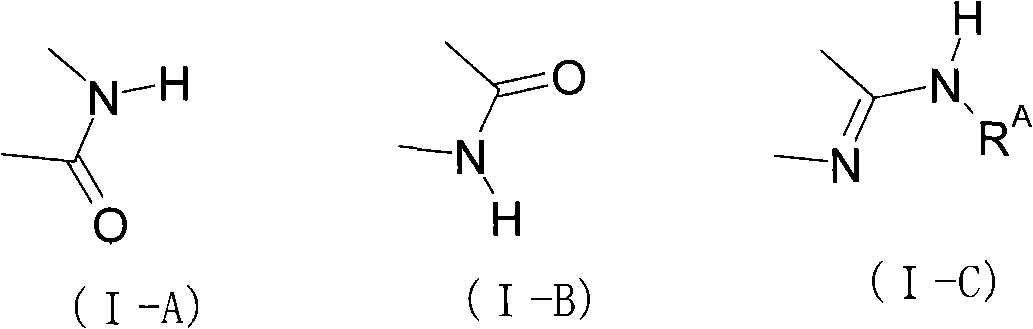
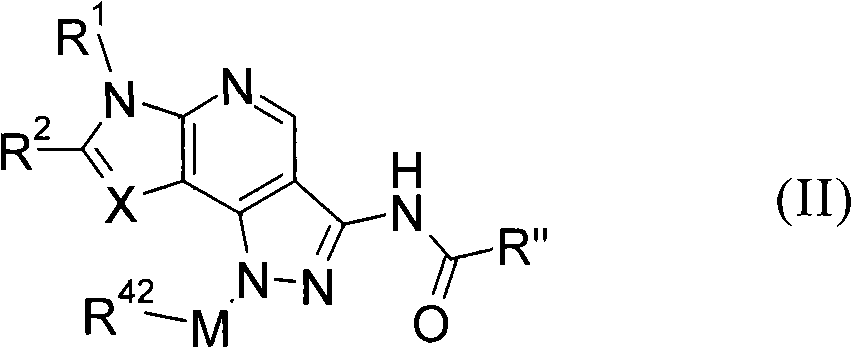
Heterocyclic Janus kinase 3 inhibitors
A kind of technology of heterocycloalkyl and heteroaryl, applied in the field of immunological disease therapeutic agents, can solve the problems such as no specific disclosed compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0163] According to a method similar to the method described in Example 1 using the corresponding starting compounds, the compounds in Preparation Examples 1-1 to 1-25 shown in the table below were prepared.
[0164] Table 2
[0165]
[0166] Table 2 (continued)
[0167]
[0168] Table 2 (continued)
[0169]
[0170] table 3
[0171] Preparation example
[0172] Table 3 (continued)
[0173] Preparation example
[0174] 1-9
preparation example 2
[0176] 3-(4-Chloro-1H-pyrrolo[2,3-b]pyridin-5-yl)-1,2,4-oxadiazole-5-carboxylic acid ethyl ester (60mg) in 1-methyl - A solution of 2-pyrrolidone (0.6ml) and 1,8-diazabicyclo[5.4.0]undec-7-ene (0.092ml) was added to glycinamide hydrochloride (34mg) , and the resulting mixture was stirred at 70 °C for 1 h. After standing to cool, with preparative HPLC (10mM NH 4 HCO 3 +NH 3 (pH=9.2)=90:10 to 20:80) directly purify the reaction solution. The active fraction was concentrated and dried to give 3-(4-chloro-1H-pyrrolo[2,3-b]pyridin-5-yl)-N-methyl-1,2,4-oxadiazole - 5-Carboxylate (23 mg) as a solid.
[0177] According to a method similar to the method described in Example 2 using the corresponding starting compounds, the compounds in Preparation Examples 2-1 to 2-26 shown in the table below were prepared.
[0178] Table 4
[0179]
[0180] Table 4 (continued)
[0181]
[0182] Table 4 (continued)
[0183]
[0184] table 5
[0185]
preparation example 3
[0187]Benzyl 4-chloro-1H-pyrrolo[2,3-b]pyridine-5-carboxylate (55mg), ethyl {[(4-amino-1-adamantyl)carbonyl]amino}acetate (54mg ) and triethylamine (80 μl) were dissolved in N-methyl-2-pyrrolidone (0.55 ml), and stirred at 180° C. for 1 hour using a microwave reaction system. Ethyl acetate and water were added to the resulting reaction liquid, and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over magnesium sulfate, and evaporated under reduced pressure. The residue was purified by silica gel column chromatography (n-hexane:ethyl acetate), the obtained compound was dissolved in dioxane (1.1ml) and methanol (1.1ml), and 10% palladium-carbon (50% wet), and the catalytic reduction reaction was carried out at ambient temperature and 1 atmospheric pressure for 4 hours. Methanol, dioxane, and 1M hydrochloric acid were added, and the precipitated solid was dissolved and filtered to remove the catalyst. The filtrate was conce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com