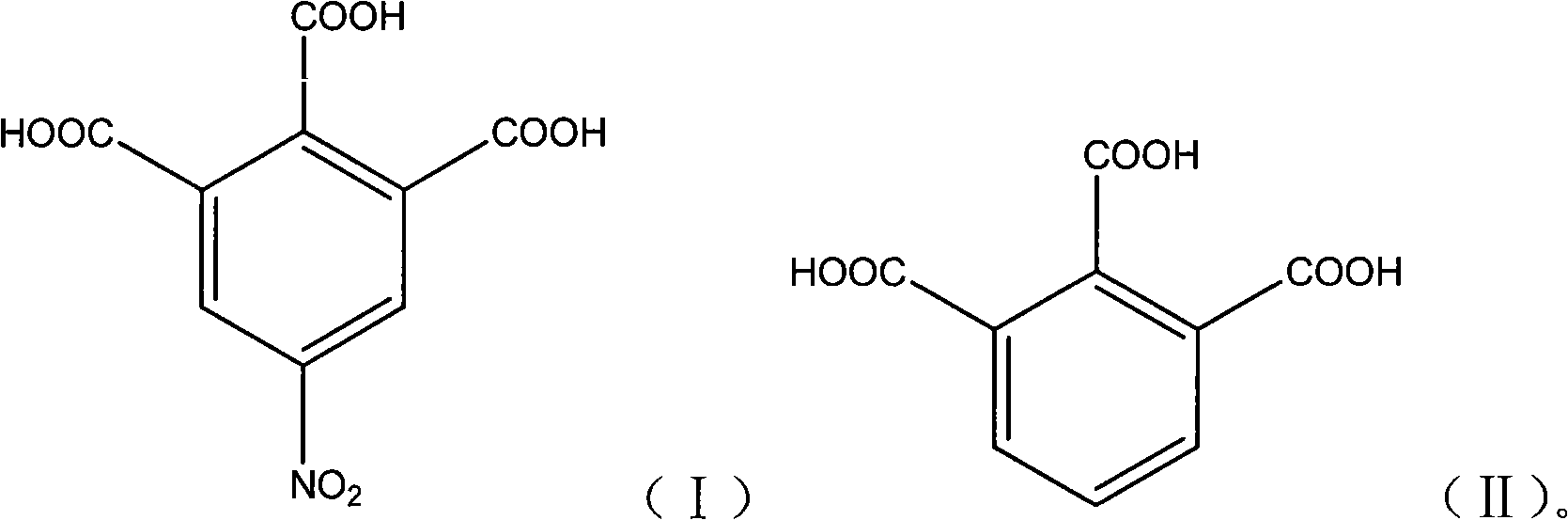
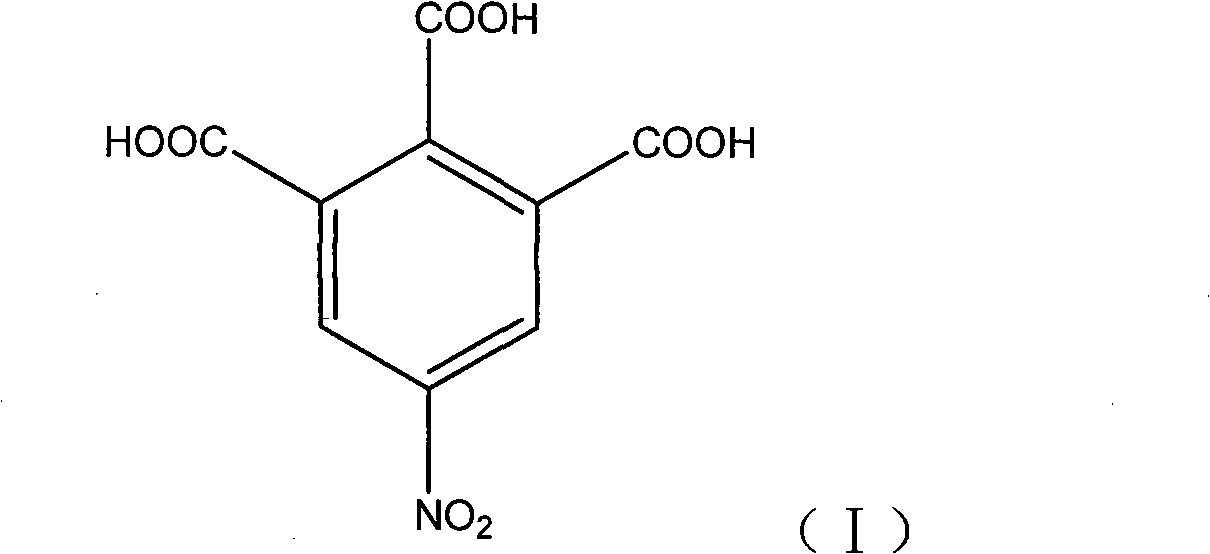
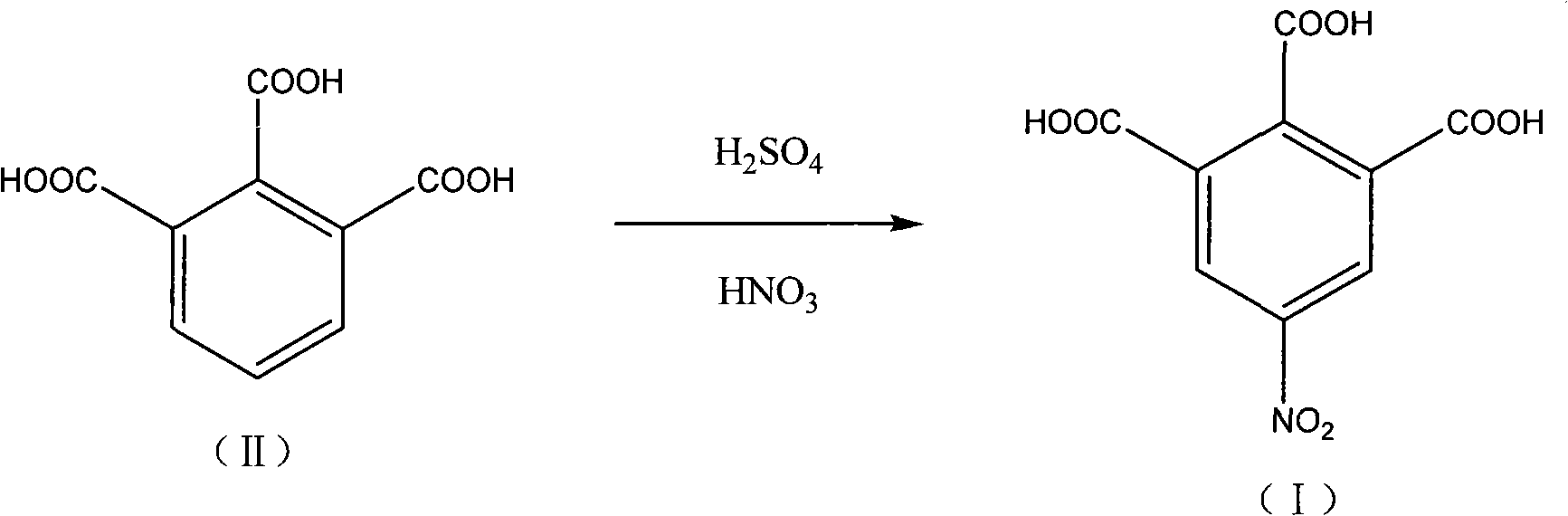
5-nitro-1,2,3-benzene tricarboxylic acid synthesizing method
A technology of trimellitic acid and nitro group, applied in the field of pharmaceutical chemical intermediate: 5-nitro-1, can solve the problems of high reaction temperature, complicated post-processing, long time and the like, and achieve high reaction yield and high product purity , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 90 g of fuming sulfuric acid into a 250 mL three-necked flask, and continue to add 60 g of fuming nitric acid and 30 g of 1,2,3-benzenetricarboxylic acid while stirring. The temperature was raised slowly, and the reaction was carried out at 50-80°C for 12 hours. After the reaction was complete, the temperature was lowered to 10°C, kept at this temperature for 5 hours, and suction filtered. The filtrate reclaims, after thick product is washed twice with sherwood oil, dry, obtain light yellow granular solid 13.0g, yield is 35.7%, HPLC purity is 97.8%, Shimadzu LC-10A, (select C18 post (4.6mm * 250mm , 5 μm) chromatographic columns; Mobile phase is 30% (v / v) aqueous methanol (gradient elution); analysis time 20min; detection wavelength is 254nm) data are shown in Table 1:
[0021] Table 1
[0022] peak number
[0023] 9
Embodiment 2
[0025] Add 180 g of fuming sulfuric acid into a 500 ml three-necked flask, and continue to add 120 g of fuming nitric acid and 30 g of 1,2,3-benzenetricarboxylic acid under stirring. The temperature rises slowly, and the reaction is carried out at 80-100°C for 24 hours. After the reaction was complete, the temperature was lowered to 10° C., kept at this temperature for 9 hours, and suction filtered. The filtrate was recovered, the crude product was washed twice with ethyl acetate, and then dried to obtain 22.0 g of a light yellow granular solid with a yield of 60.4% and an HPLC purity of 98.3%.
Embodiment 3
[0027] Add 150 g of fuming sulfuric acid into a 500 ml three-necked flask, and continue to add 80 g of fuming nitric acid and 30 g of 1,2,3-benzenetricarboxylic acid under stirring. The temperature was raised slowly, and the reaction was carried out at 100-130° C. for 12 hours. After the reaction was complete, the temperature was lowered to 10°C, kept at this temperature for 5 hours, and suction filtered. The filtrate was recovered, the crude product was washed twice with acetone, and then dried to obtain 28.2 g of a light yellow granular solid with a yield of 77.5% and an HPLC purity of 98.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com