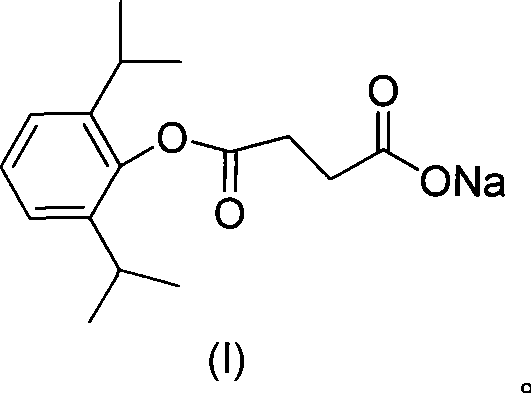
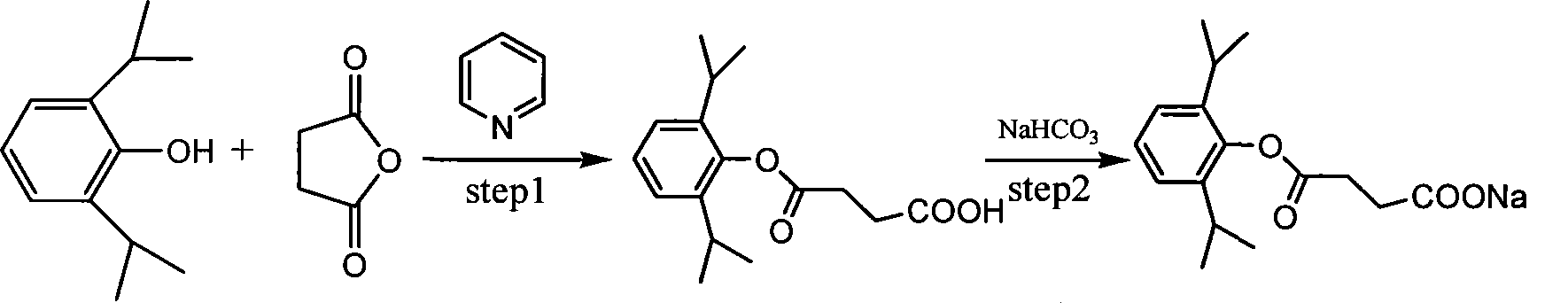
Use of propofol succinic acid monoester sodium salt in preparing anti-tumor medicine
A technology of propofol succinate and monoester, applied in the application field of propofol succinate monoester sodium salt in the preparation of antitumor drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0009] Embodiment 1, the preparation of propofol succinate monoester sodium salt
[0010] Put 4.11g of succinic anhydride and 10ml of pyridine in a 50ml three-neck flask, mix and stir for 0.5h, add 2g of propofol raw material, protect with nitrogen (propofol is easy to oxidize in an alkaline environment), heat to reflux, and the temperature is 115.5 °C, after refluxing for 1 h, the color of the solution gradually became darker. Maintain the temperature and continue to reflux for 15 hours. According to TLC detection, the raw material point basically disappears, and the reaction can be considered complete.
[0011] Pyridine was sucked dry under reduced pressure (70°C, 3 mm) to obtain a brown solid, which was dissolved by adding NaHCO3 aqueous solution, and adjusted to PH=1-2 with hydrochloric acid, a light gray solid precipitated in the solution, filtered the precipitated solid, and dried in vacuo to obtain isopropyl The crude product of phenolsuccinate monoester was 3.16g, the...
Embodiment 2
[0012] Example 2 Effect of Propofol Succinate Monoester Sodium Salt on Human Breast Cancer Cells
[0013] Test drug: Propofol succinate monoester sodium salt
[0014] method:
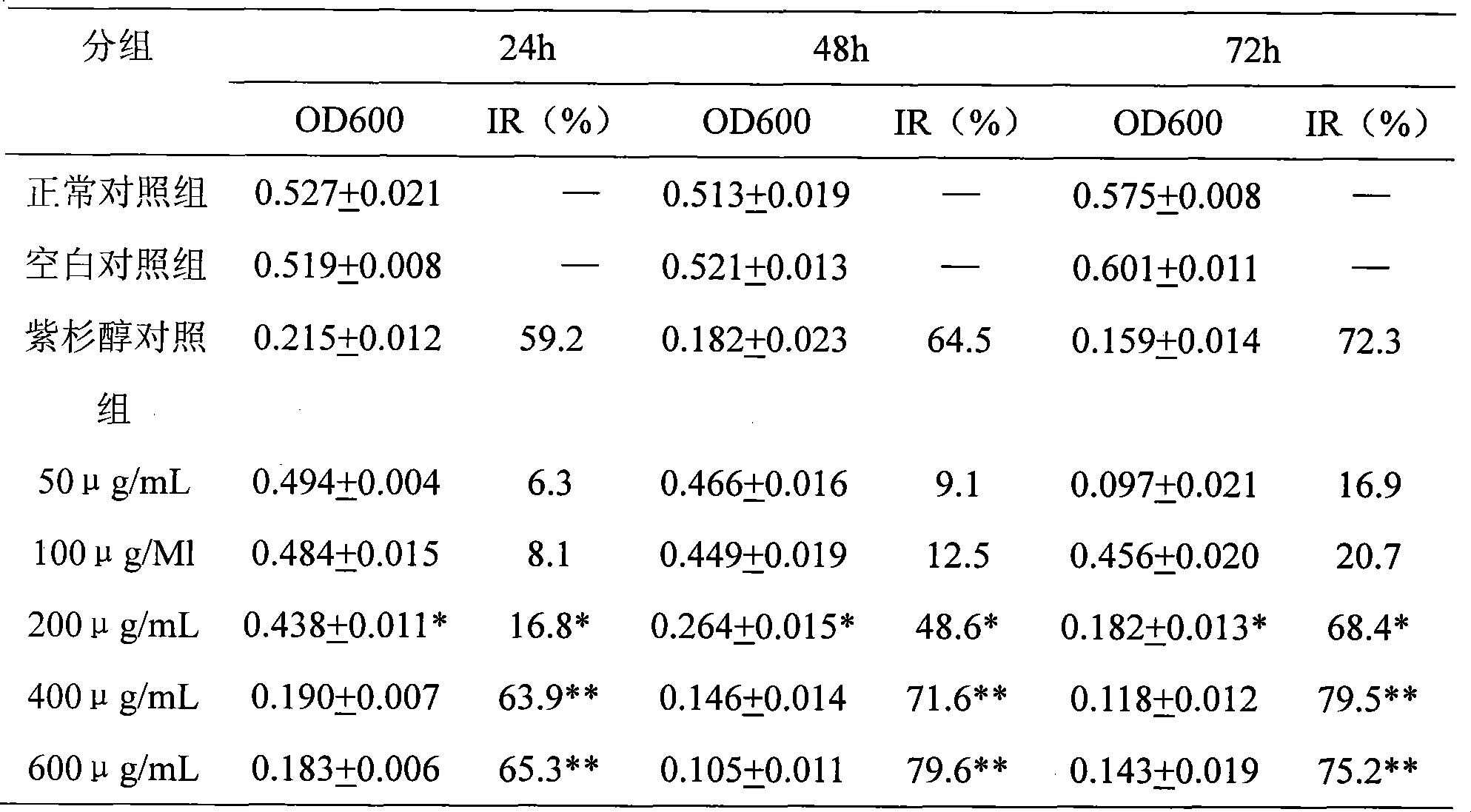
[0015] The human breast cancer cell line MDA-MB-231 was cultured in vitro, and the experiment was divided into 8 groups: ① normal control group ② blank control group ③ paclitaxel control group, propofol succinate monoester sodium salt intervention group with different concentrations ④ 50 μM ⑤ 100 μM ⑥ 200 μM ⑦ 400 μM ⑧ 600 μM. After the intervention, each experimental group continued to culture for 24, 48, and 72 hours before investigation. MTT method was used to select the optimal cell inoculation concentration, culture time and optimal inhibitory concentration, and calculate its IC50 value.
[0016] Results: Changes in OD value and inhibition rate of MDA-MB-231 cells treated with propofol succinate monoester sodium salt for 24h, 48h, and 72h (n=4)
[0017]
[0018] Note: 400μg / mL and 600μg / mL co...
Embodiment 3
[0024] Example 3 Effect of Propofol Succinate Monoester Sodium Salt on Human Neuroblastoma Cells and Liver Cancer Cells
[0025] method:
[0026] The concentrations of hydroxycamptothecin in the neuroblastoma cell group and the liver cancer cell group were 100 μg / mL and 80 μg / mL, respectively, and the method was the same as
[0027] result:
[0028] As shown in the table: ① neuroblastoma cells: 50 μM, 100 μM group had no significant difference in absorbance value from normal control group and blank control group (P>0.05), and the absorbance value of 200 μM, 400 μM, 600 μM group was compared with 50 μM, 100 μM group There is a very significant difference (P0.05), but there was a significant difference between the 400μM and 600μM groups (P0.05), but it was very significant compared with the 600μM group (P<0.01). Its optimum inhibitory concentration is 200μg / mL, and its IC50 is 268.2μg / mL.
[0029] ② Liver cancer cells: There was no significant difference in absorbance between...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com