Method and equipment for preparing terephthalic acid by air oxidation of p-xylene
A technology of terephthalic acid and p-xylene, which is applied in the field of preparing terephthalic acid by air oxidation of p-xylene, can solve the problems that it is not suitable for continuous industrial production, and the content of terephthalic acid cannot meet the requirements of industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
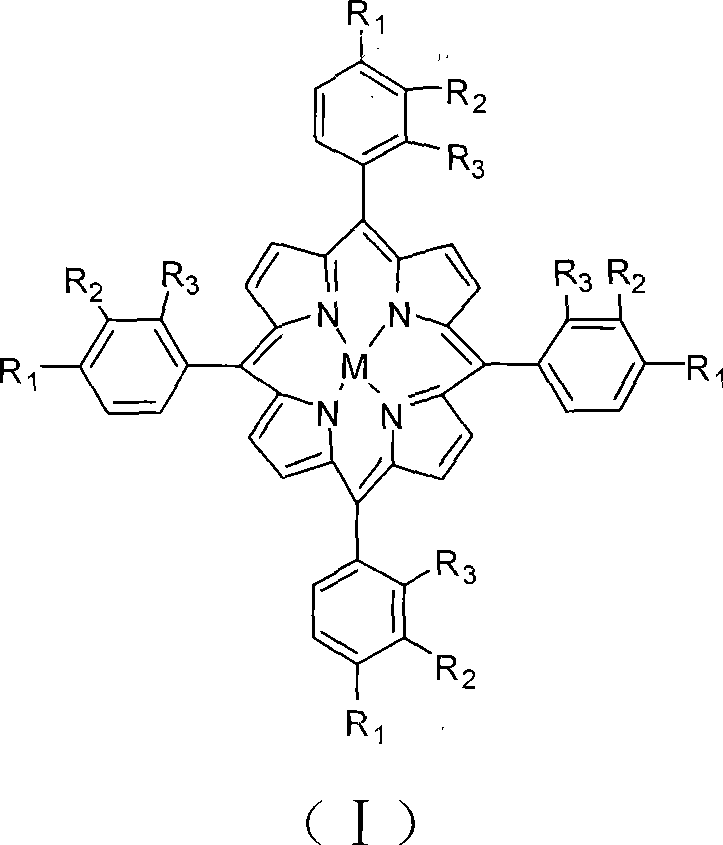
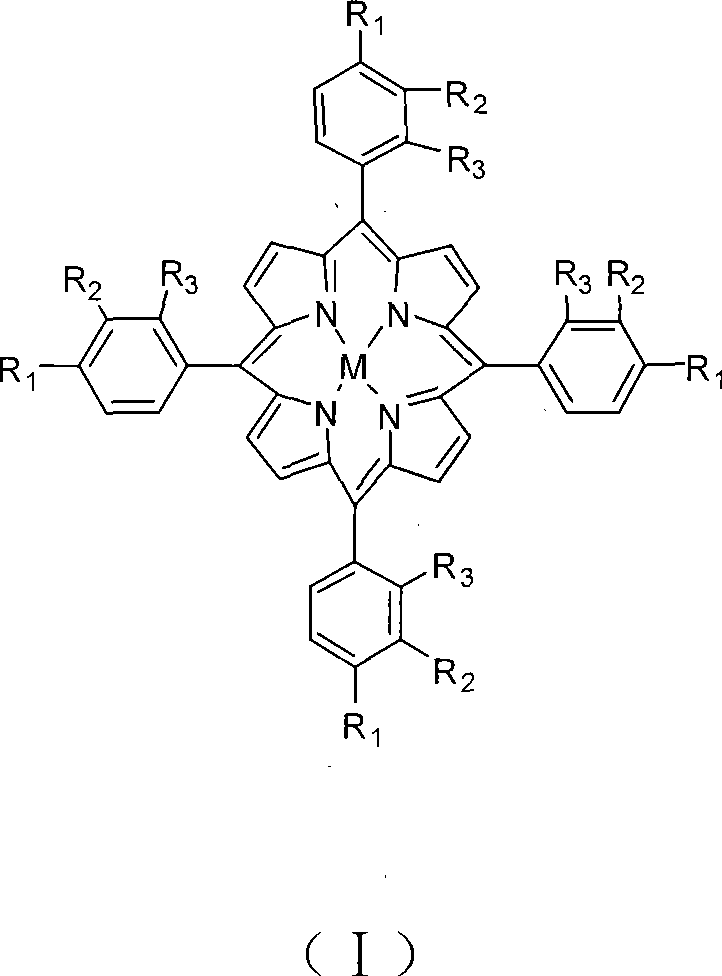
[0016] The reaction equipment includes a reaction kettle with mechanical stirring and air distributor and a crystallization tank, and the oxidation reaction kettle and the crystallization separation tank are connected by pipelines and solid pumps at the same time. The production process is as follows: at 180°C, the metalloporphyrin dissolved in 2PPM structural formula (I) is introduced, R 1 = R 2 = R 3 =H, the p-xylene of M=Co, pass 18atm air through the gas distributor into the bottom of the stirred reactor, control the p-xylene flow rate so that the residence time is 100 minutes, control the air flow rate so that the tail oxygen content is no more than 6% . The reaction solution in the upper part of the stirring reactor overflows into the crystallization separation tank, and the solid suspension in the lower part of the stirring reaction tank enters the crystallization separation tank through a pump. The crystallization knockout tank was maintained at 155°C and 1 atm. Te...
Embodiment 2
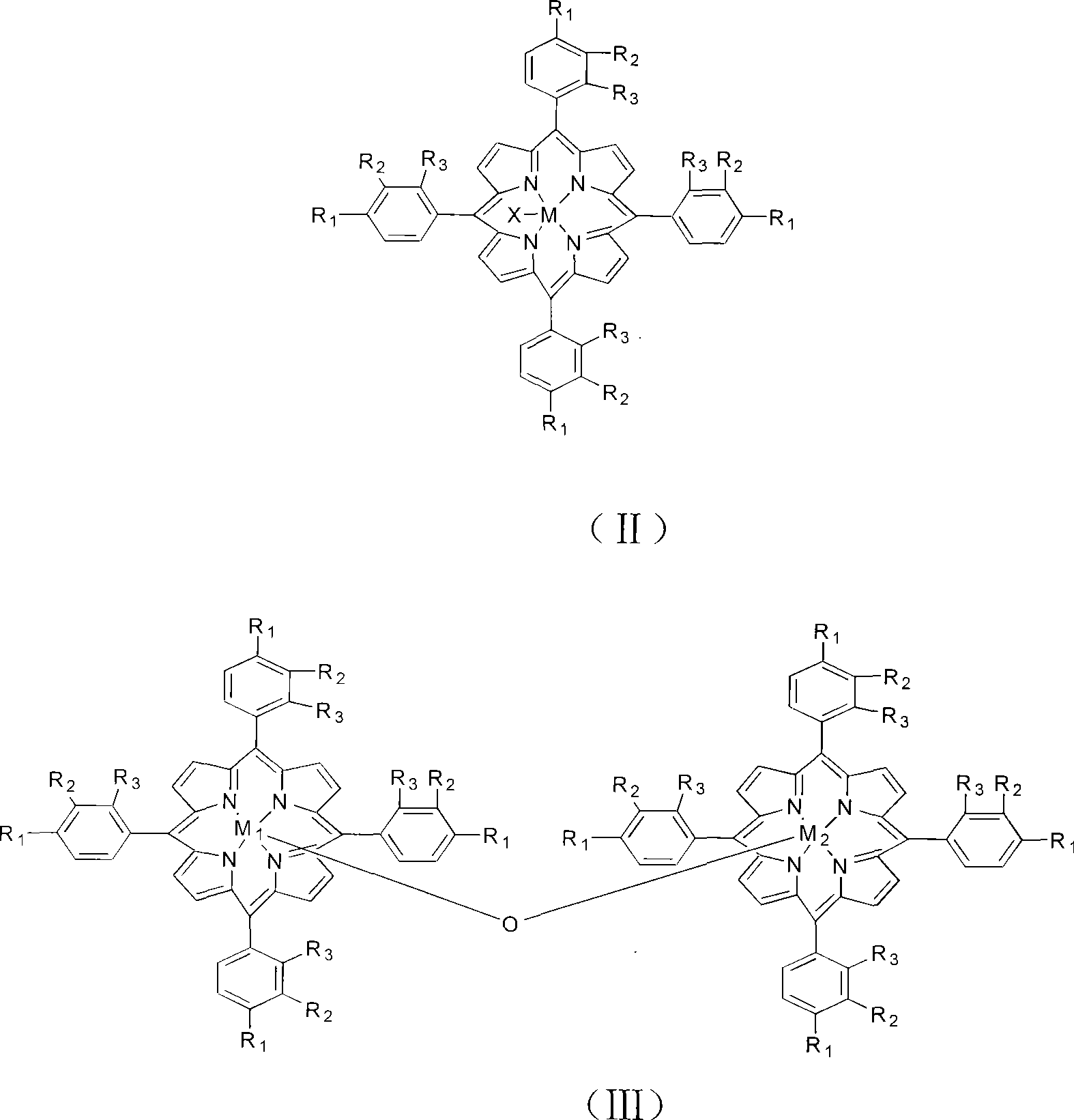
[0018] The reaction equipment includes 2 reaction kettles with electric stirring and air distributor and 1 crystallization tank. The oxidation reaction kettle and the crystallization separation tank are connected by pipelines and solid pumps at the same time. The production process is as follows: at 185°C, the metalloporphyrin dissolved in 8PPM structural formula (II) is introduced, R 1 =CH 3 , R 2 = R 3 =H, M=Fe, p-xylene whose ligand X is Cl, pass 16atm oxygen-containing 18% oxygen-poor air through the gas distributor into the bottom of the stirred reactor 1, and stir the reaction liquid in the upper part of the reactor 1 Flow into the stirred reactor 2 through the overflow, the suspended reaction liquid in the lower part of the stirred reactor 1 is pumped into the stirred reactor 2 through a pump, and the oxygen-depleted air containing 18% oxygen is introduced into the stirred reactor 2. The p-xylene flow rate was controlled to give a residence time of 120 minutes. Cont...
Embodiment 3
[0020] The reaction equipment includes 3 reaction kettles with magnetic stirring and air distributor and 1 crystallization tank, and the oxidation reaction kettle and the crystallization separation tank are connected by pipelines and solid pumps at the same time. The production process is as follows: at 195 ° C, the metalloporphyrin dissolved in 50PPM structural formula (III) is introduced, R 1 =OCH 3 , R 2 = R 3 = H, M 1 = M 2=Mn p-xylene, 20atm oxygen-containing 23% oxygen-enriched air is passed through the gas distributor to the bottom of the stirred reactor 1, and the reaction liquid in the upper and lower parts of the reactor 2 enters the stirred reactor through the overflow and the pump respectively 2, feed oxygen-enriched air containing 23% oxygen into the stirred reactor 2, and the reaction solution in the upper and lower parts of the stirred reactor 2 enters the bottom of the stirred reactor 3 through overflow and pump respectively, and passes through the stirred ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com