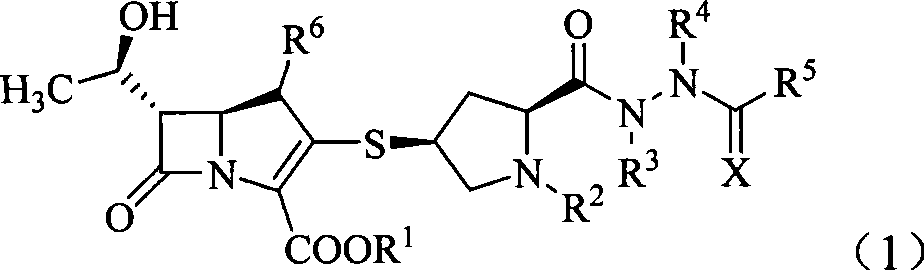
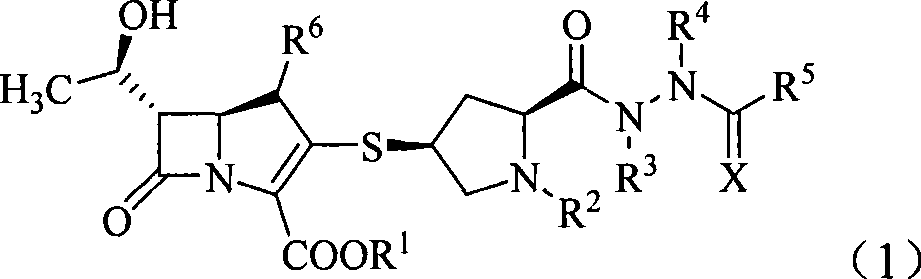
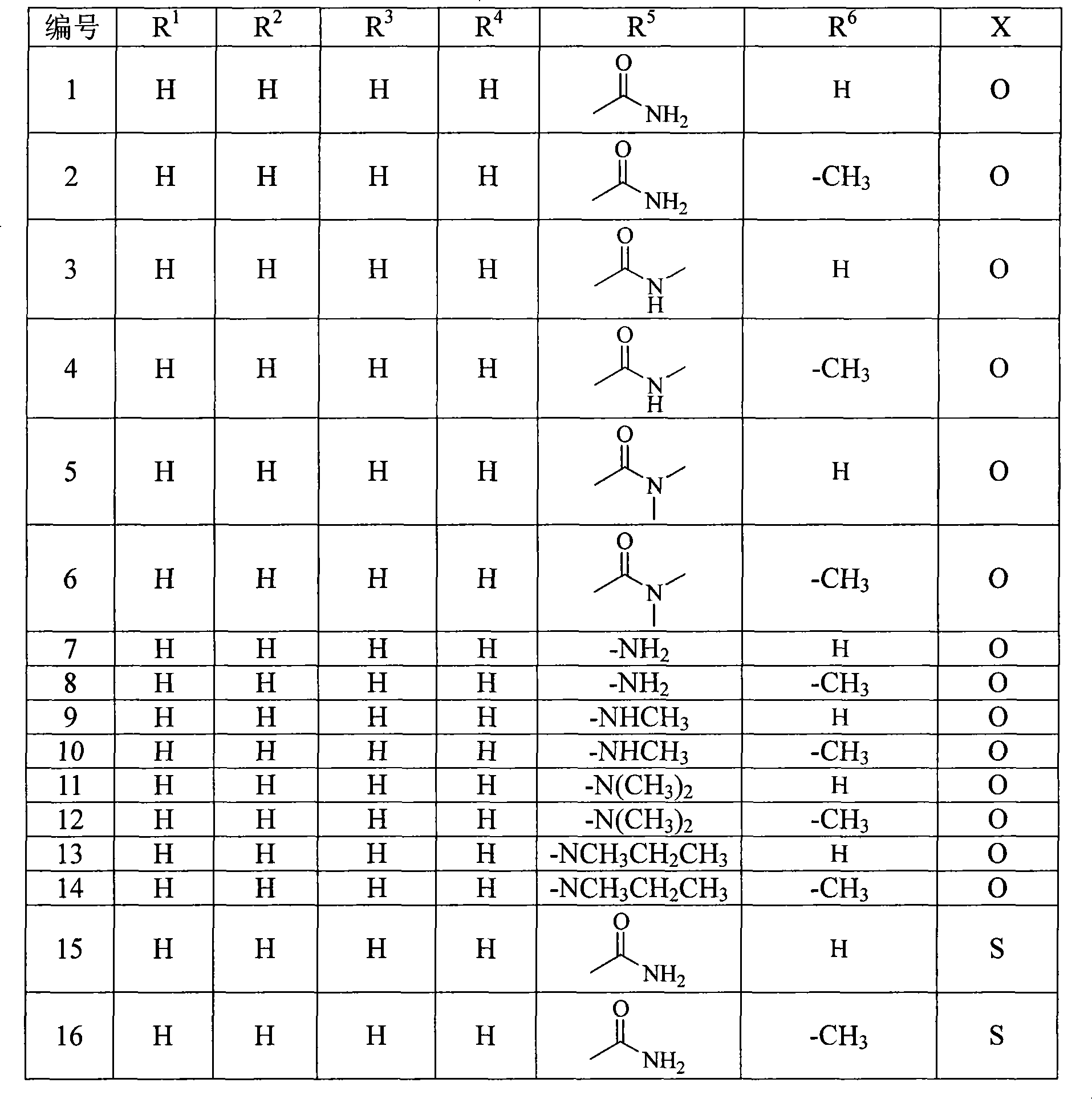
Penem derivant containing sulfhydryl pyrrolidine formhydrazide
A methyl and amino technology, applied in the field of penem derivatives containing mercaptopyrrolidine hydrazide, which can solve the problems of low clinical utilization and short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Example 1 (2S, 4S)-4-mercapto-2-formyl [(2-amino-2-oxoacetyl) hydrazino] -1- (tert-butoxycarbonyl) pyrrolidine preparation
[0084] Add (2S, 4S)-4-acetylthio-2-carboxy-1-(tert-butoxycarbonyl)pyrrolidine 8.7g (30mmol) and 100ml of anhydrous tetrahydrofuran to the dry reaction flask, under nitrogen protection, in Add 6.5g (40mmol) of 1,1-carbonyldiimidazole at room temperature, react for 0.5h, add 4.1g (40mmol) of 2-amino-2-oxoacetylhydrazine in 100ml of tetrahydrofuran solution below 0°C, and continue the reaction for 0.5h, Then 40ml of 1mol / L hydrochloric acid was added dropwise, extracted with ethyl acetate (50ml×2), the organic phase was washed with water and saturated sodium chloride solution successively, concentrated under reduced pressure, the residue was added with 100ml of 3mol / L hydrochloric acid, stirred for 2h, and The dilute alkaline solution was adjusted to be basic, and a solid was precipitated, which was recrystallized to obtain 6.1 g of a solid, with...
Embodiment 2
[0085] Example 2 Preparation of (2S, 4S)-4-mercapto-2-formyl [(4-methyl-thiourea-1-yl) amino]-1-(tert-butoxycarbonyl)pyrrolidine
[0086] Refer to Example 1 for the specific preparation method. Throw (2S, 4S)-4-acetylthio-2-carboxy-1-(tert-butoxycarbonyl)pyrrolidine 8.7g (30mmol) and 4-methylthiosemicarbazide 4.2g (40mmol). 6.3 g of the product was obtained, yield: 63.2%.
Embodiment 3
[0087] Example 3 Preparation of (2S, 4S)-4-mercapto-2-formyl (guanidine-1-yl) amino-1-(tert-butoxycarbonyl) pyrrolidine
[0088] Refer to Example 1 for the specific preparation method. Throw (2S, 4S)-4-acetylthio-2-carboxy-1-(tert-butoxycarbonyl)pyrrolidine 8.7g (30mmol) and aminoguanidine 3.0g (40mmol). 5.9 g of the product was obtained, yield: 65.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com