Novel vaccine carrier
A vaccine carrier and vaccine technology, applied in liposome delivery, antibody medical components, viruses/phages, etc., can solve the problems of not rising antibody titers and ineffective induction of immune responses, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1: the making of succinylated polyglycidol (SucPG) liposome
[0060] As liposomes containing SucPG, lipids formed to dipalmitoylphosphatidylcholine (DPPC) (Sigma) and dioleylphosphatidylethanolamine (DOPE) (Sigma) in a molar ratio of 1:1 (10 micromoles each) In the lipid combination, SucPG with a weight ratio of 10%, 20% or 30% of lipid was added to produce three kinds of SucPG-containing liposomes with different SucPG concentrations. The SucPG used in this embodiment has a n-decyl group with 10 carbon atoms as an alkyl group, which is synthesized according to the method described in the literature (KonoK. et al., J.Controlled Release, 68, 225-235 (2000 ); Kono K. et al., Biochim.Biophys.Acta (Biochemistry and Biophysics Acta), 1325, 143-154 (1997); or Kono K. et al., Biochim.Biophys.Acta (Biochemistry and Biophysics Acta ), 1193, 1-9 (1994)). Specifically, polyglycidyl acetate was prepared by reacting polyepichlorohydrin in dimethylformamide at 175°C for 6 h...
Embodiment 2
[0062] Embodiment 2: the study of the immune response when the vaccine formed by succinylated polyglycidyl (SucPG) liposomes is inoculated through the mucosa
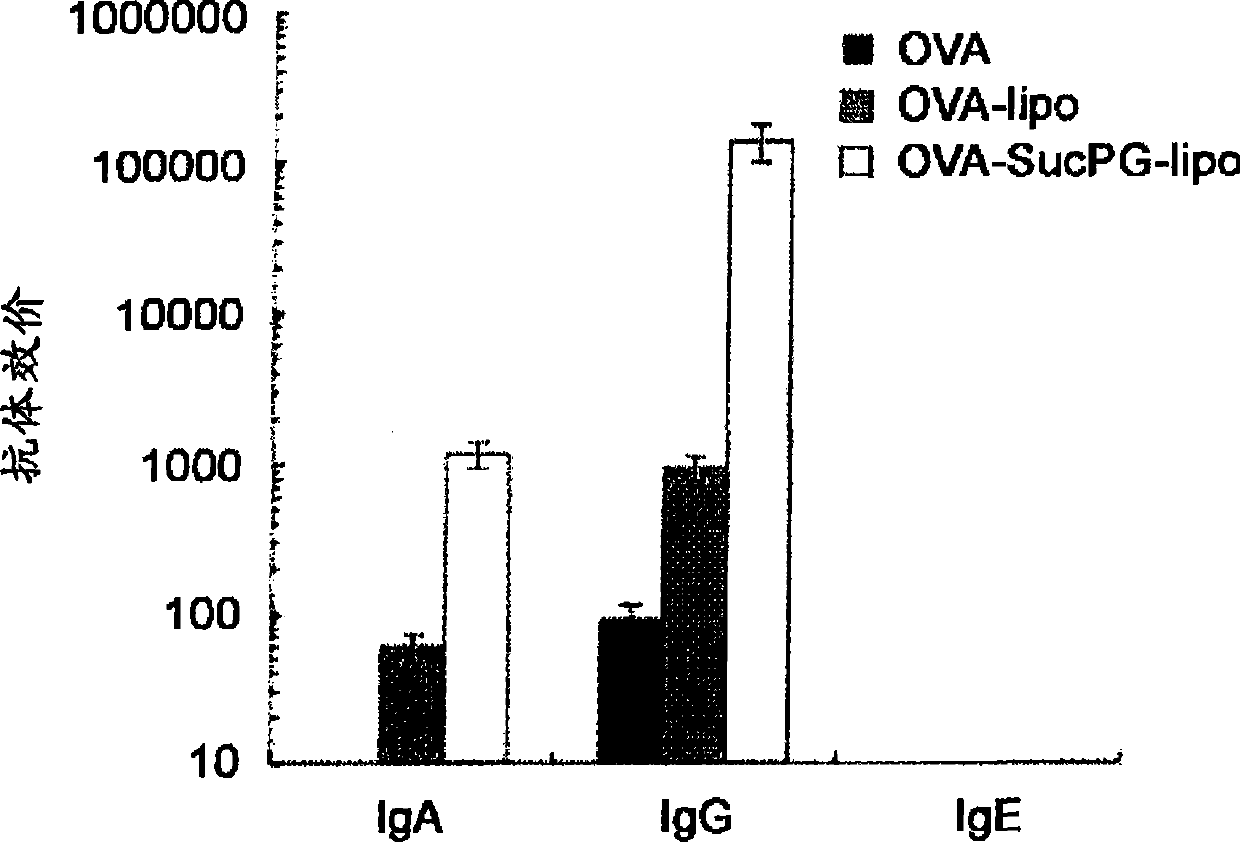
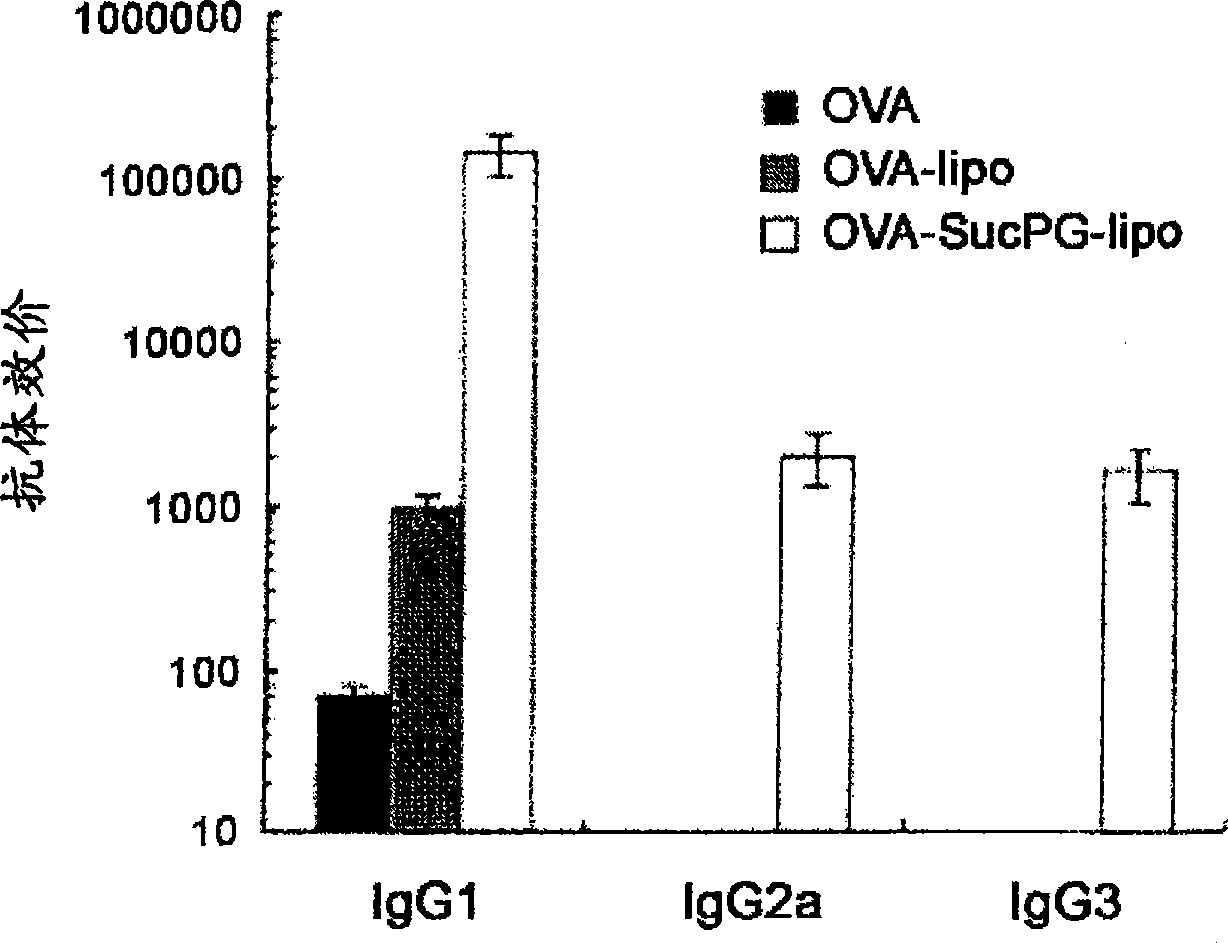
[0063] The purpose of this embodiment is: the immune response of the vaccine formed by the liposome containing SucPG made in Example 1 when inoculated through the mucosa, and the vaccine formed by the liposome not containing SucPG are comparatively studied.
[0064] The vaccine formed by the liposome containing SucPG was prepared according to the method described in Example 1. On the other hand, as a vaccine formed from liposomes not containing SucPG, OVA was encapsulated in a lipid composition with a molar ratio of DPPC:DOPE of 1:1, thereby making multilamellar liposomes (OVA - Vaccines formed from liposomes) (MLV).
[0065] The above-mentioned OVA-SucPG-liposome-formed vaccine, the OVA-liposome-formed vaccine, and the OVA-only vaccine were inoculated to BALB / c mice in a nasal manner, separated by 7 Inoculate twice a...
Embodiment 3
[0072] Example 3: Induction of cellular immune response brought about by mucosal administration of vaccines formed by liposomes containing SucPG
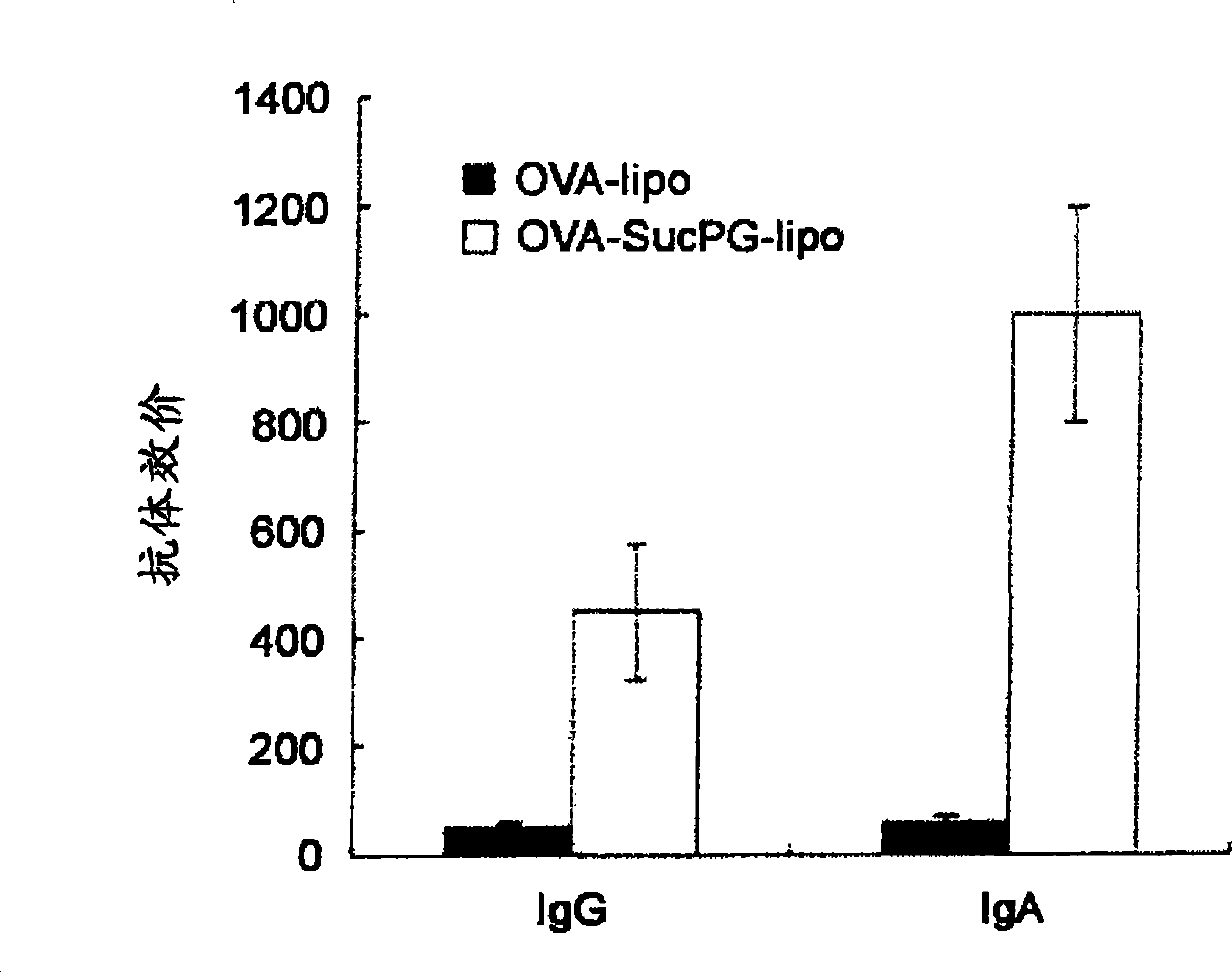
[0073] In Example 2, it was shown that the use of a vaccine formed by liposomes containing SucPG, through mucosal inoculation of antigens for immunization, has the possibility of inducing a cellular immune response. Therefore, in this example, the use of liposomes containing SucPG The ability of liposomes to form a cellular immune response to vaccines was studied.
[0074] The vaccine formed by the OVA-SucPG-liposome prepared in Example 1 was inoculated nasally to BALB / c mice, and inoculated twice at intervals of 7 days, and the dosage of OVA each time was 100 μg / mouse. Seven days after the final inoculation, the mice were sacrificed, the spleens were removed, and spleen lymphocytes were purified by density gradient centrifugation.
[0075] Using TRIzol TM (Invitrogen) Total RNA was extracted from purified splenic lymphocytes, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com