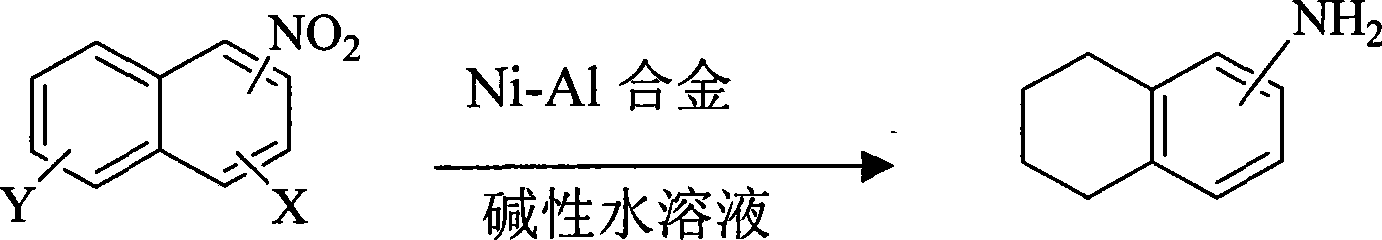
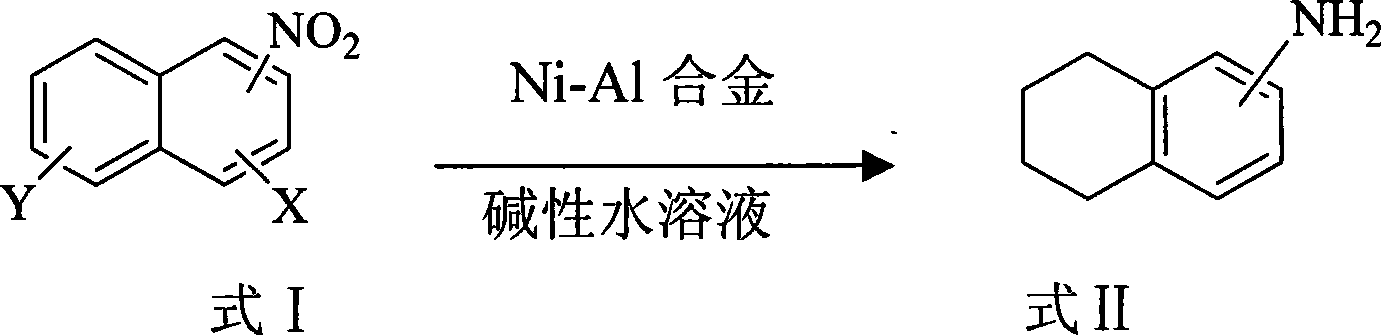
Synthetic method of tetralin amines compounds
A tetrahydronaphthylamine and synthetic method technology, applied in the direction of organic chemistry, etc., can solve the problems of harsh experimental conditions, environmental pollution, etc., achieve the effects of reducing the elimination of three industrial wastes, avoiding the use of organic solvents, and low production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] In a three-necked reaction flask equipped with a thermometer and a stirrer, add 1-nitronaphthalene (8.7 g, 0.05 mol), water (100 mL), 1:1 (W / W) Ni- Al alloy (4.35g), then heated to 60°C with stirring. Then 1 wt% aqueous sodium hydroxide solution (100 ml) was added dropwise for 1 hour. The reaction was directly followed by TLC to complete the reaction of 1-nitronaphthalene (5h). After the reaction, the reaction solution was cooled to room temperature (20-25° C.), left to filter, extracted the aqueous phase with dichloromethane organic solvent, concentrated and evaporated the organic solvent to obtain 1-tetrahydronaphthylamine with a yield of 76% and a purity of 98.2 % (HPLC).
Embodiment 2
[0018] In a three-necked reaction flask equipped with a thermometer and a stirrer, add 2-nitronaphthalene (8.7 g, 0.05 mol), water (80 mL), 1:1 (W / W) Ni- Al alloy (8.7g), then stirred and heated to 85°C. Then 20wt% cesium hydroxide aqueous solution (80ml) was added dropwise for 1 hour. The reaction was directly followed by TLC to complete the reaction of 1-nitronaphthalene (4h). After the reaction, the reaction liquid was cooled to room temperature, stood and filtered, extracted the aqueous phase with dichloromethane organic solvent, concentrated and evaporated the organic solvent to obtain 2-tetrahydronaphthylamine with a yield of 80% and a purity of 98.8% (HPLC).
Embodiment 3
[0020] In a three-necked reaction flask equipped with a thermometer and a stirrer, add 1-nitronaphthalene (8.7g, 0.05mol), water (500mL), 1:1 (W / W) Ni -Al alloy (43.5g), then stirred and heated to 50°C. Then 10wt% lithium hydroxide aqueous solution (500ml) was added dropwise for 1 hour. The reaction was directly followed by TLC to complete the reaction of 1-nitronaphthalene (3h). After the reaction, the reaction solution was cooled to room temperature (20-25° C.), left to filter, extracted the aqueous phase with dichloromethane organic solvent, concentrated and evaporated the organic solvent to obtain 1-tetrahydronaphthylamine with a yield of 86% and a purity of 99.2 %(HPLC)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com