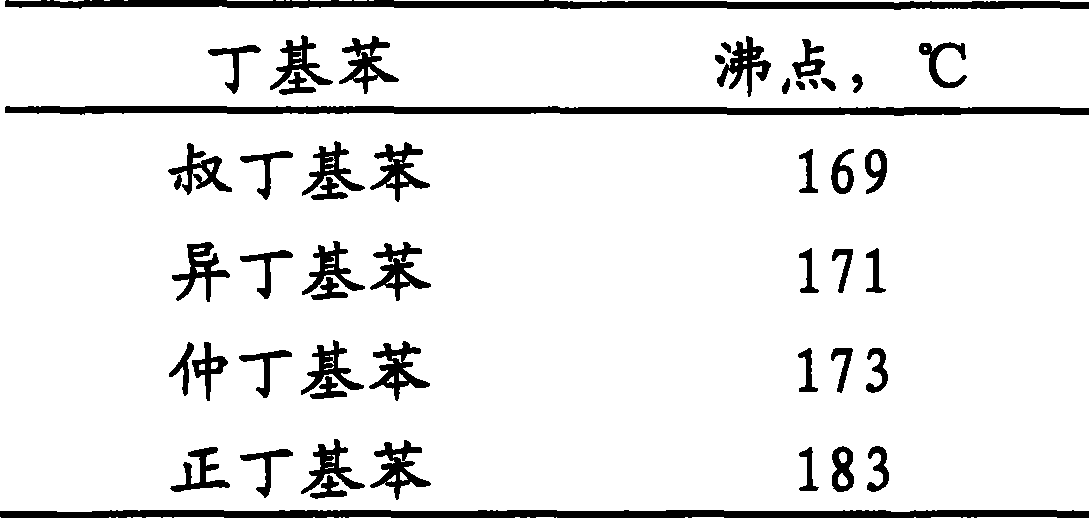
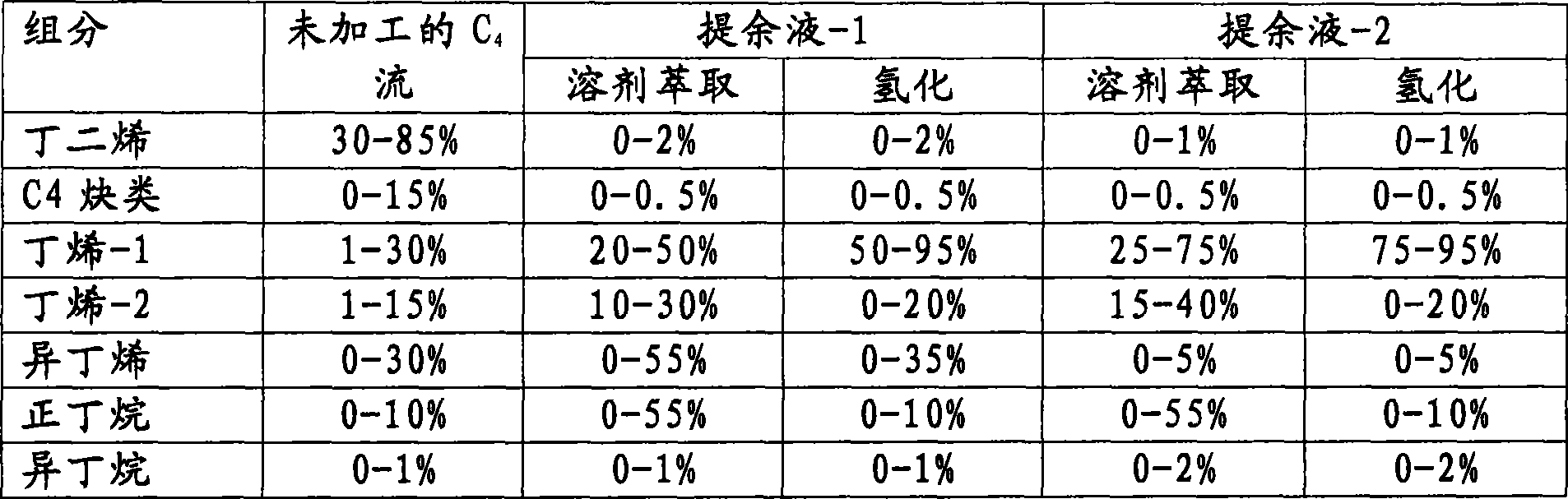
Process for producing sec-butylbenzene
A sec-butylbenzene and butene technology, applied in chemical instruments and methods, carbon-based compound preparation, organic compound preparation, etc., can solve the problems of consumption and damage to sec-butylbenzene selectivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
Embodiment 1 (comparison)
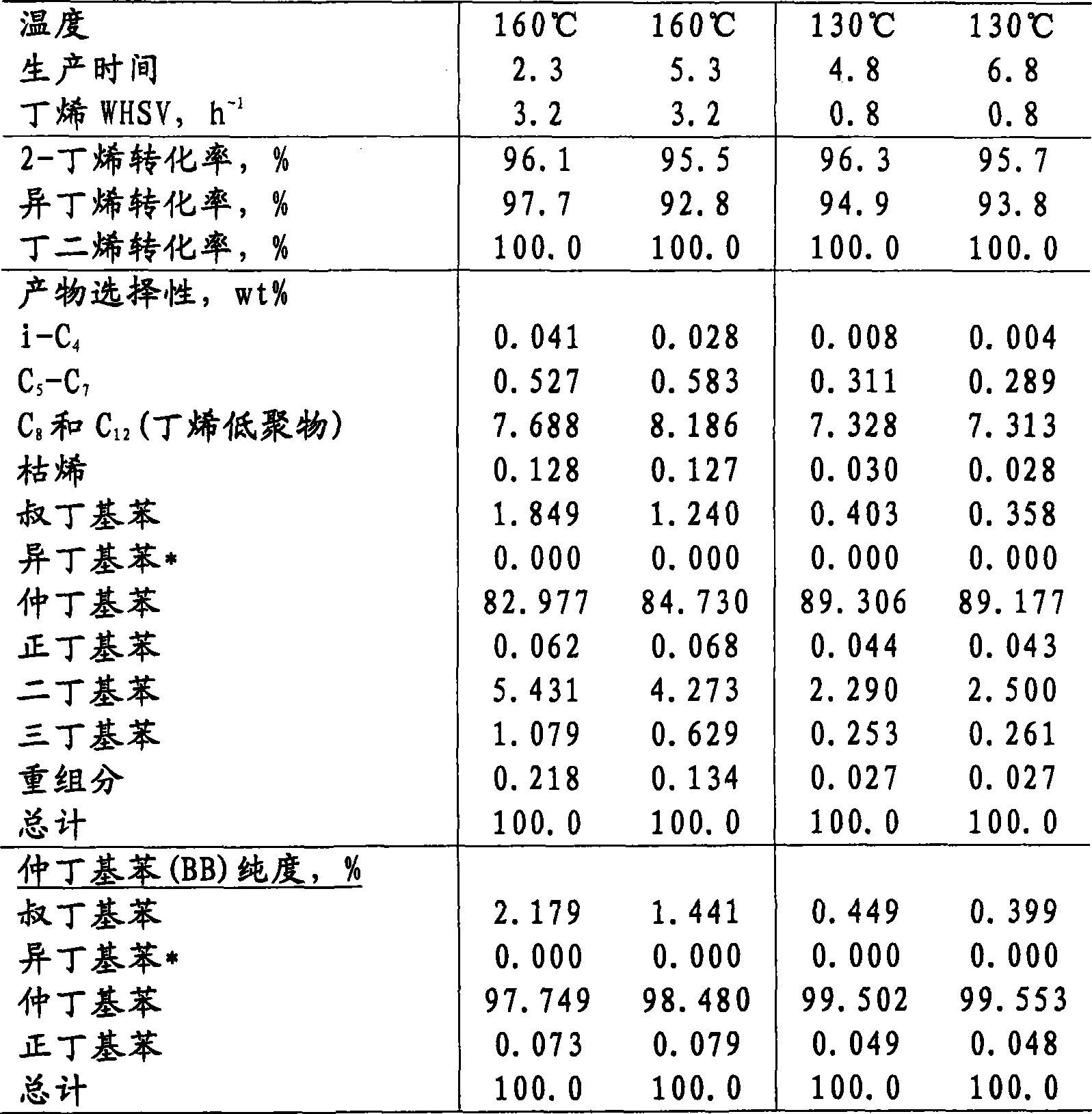
Synthesis of sec-butylbenzene by MCM-49 at 160℃
[0054] A 0.5 g MCM-49 catalyst sample (1.3 mm [1 / 20"] quadrulobe extrudate of 60% MCM-49 / 40% Versal 200 alumina binder, cut into 1.3 mm [1 / 20 "] length) for benzene alkylation with a mixed butene feed having the following composition: 53.4% cis-butene, 41.2% trans-butene, 4.6% isobutene, 0.5% butadiene, 0.1% n-butene alkanes and 0.2% other substances. The catalyst was diluted to 3 cc with sand and loaded into a 4.76 mm (3 / 16") OD isothermal downflow fixed bed reactor. The catalyst was dried at 150° C. and 1 atmosphere with 100 cc / min of flowing nitrogen for 2 hours. Turn off the nitrogen and feed benzene into the reactor at 60 cc / hr until the reactor pressure increases to 300 psig (2170 kPa).Then the benzene flow is reduced to 7.63 cc / hr (6.67 WHSV), and the mixed butene feed is fed at 2.57 cc / hr was introduced by a syringe pump. The reactor temperature was adjusted to 160 °C. The benzene / butene m...
Embodiment 2
Synthesis of sec-butylbenzene by MCM-49 at 130℃
[0055] The procedure of Example 1 was repeated, but with 2.0 g of the MCM-49 catalyst used in Example 1, and the reactor temperature was adjusted to 130°C and maintained at 130°C throughout the run. Production was carried out with the catalyst at 0.8 WHSV of butenes at 94%+2-butene conversion for 8 days and at 2.4 WHSV for 1 day at 69-72% conversion. The relative activity of MCM-49 based on first-order butene conversion was 0.2. Representative data are listed in Table 2.
Table 2 Production of sec-butylbenzene at 130°C by feeding MCM-49 and mixed butenes
Production time 4.8 6.8 8.8 Butene WHSV, h -1 0.8 0.8 2.4 2-butene conversion, % 96.3 95.7 71.3 Isobutene conversion, % 94.9 93.8 62.9 Butadiene conversion, % 100.0 100.0 100.0 Product selectivity, wt% i-C 4 0.008 0.004 0.004 C 5 -C 7 0.311 0.289 0.311 C 8 and C 12 (butene oligomer) 7.328 7...
Embodiment 3
Synthesis of sec-butylbenzene with MCM-22 at different temperatures
[0057] 1.0 g of MCM-22 catalyst sample (65 wt% MCM-22 / 35% alumina binder 1.6 mm [1 / 16”] diameter cylindrical extrudate, cut to 1.6 mm [1 / 16”] length) For benzene alkylation using the mixed butene feed of Example 1 and following the procedure of Example 1 (reactor temperature 160°C). The catalyst was produced at 1.6 WHSV butene at 98% 2-butene conversion for 6 days, at 4.8 WHSV at 80% conversion for 1 day, at 7.2 WHSV at 62% conversion for 1 day, then again at 1.6 WHSV at 97% conversion for 4 days. Representative data are listed in Table 4. The relative activity of MCM-22 based on primary butene conversion was 0.5.
[0058] The same 2.3 g sample of MCM-22 catalyst (65% MCM-22 / 35% alumina binder) was used for benzene alkylation using the mixed butene feed of Example 1 and following the procedure of Example 2. Method (reactor temperature 130°C). Butenes were produced at 0.43 WHSV for 4 days at 94%+ conversi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com