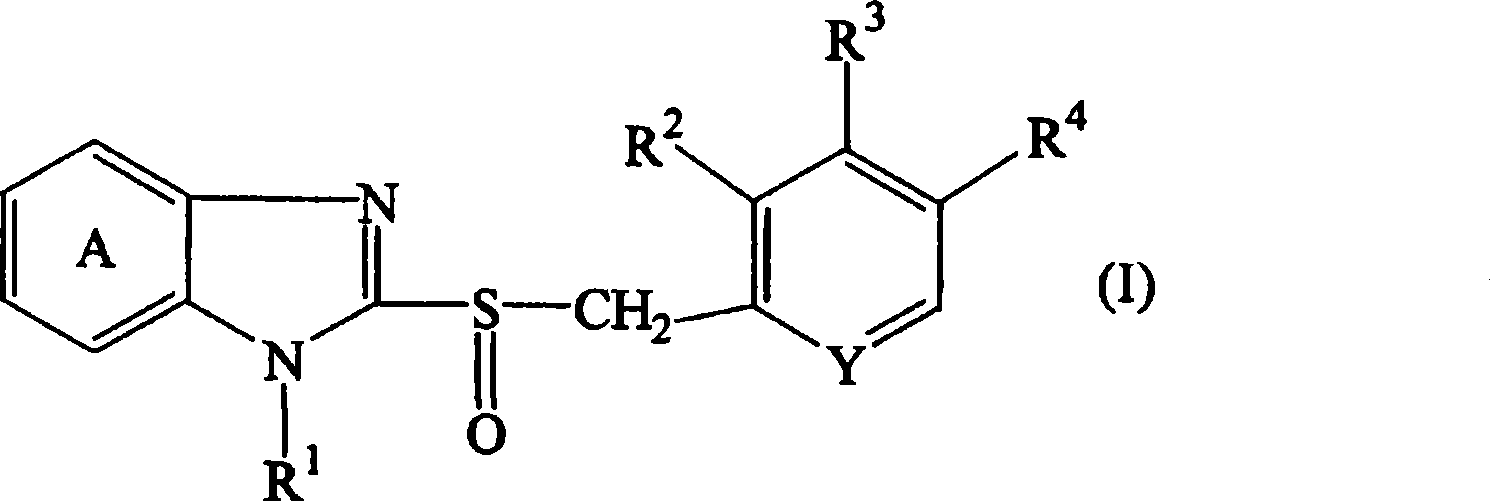
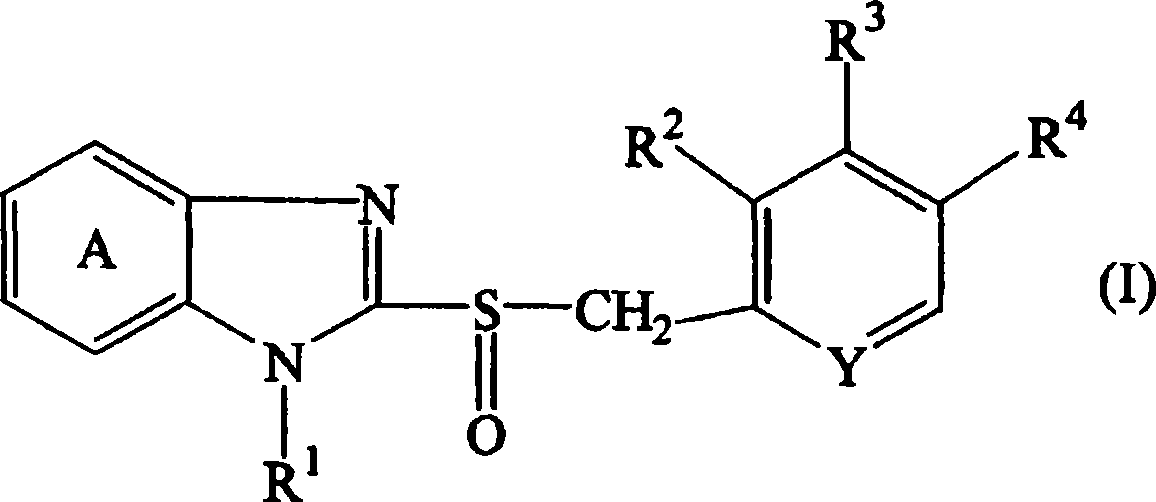
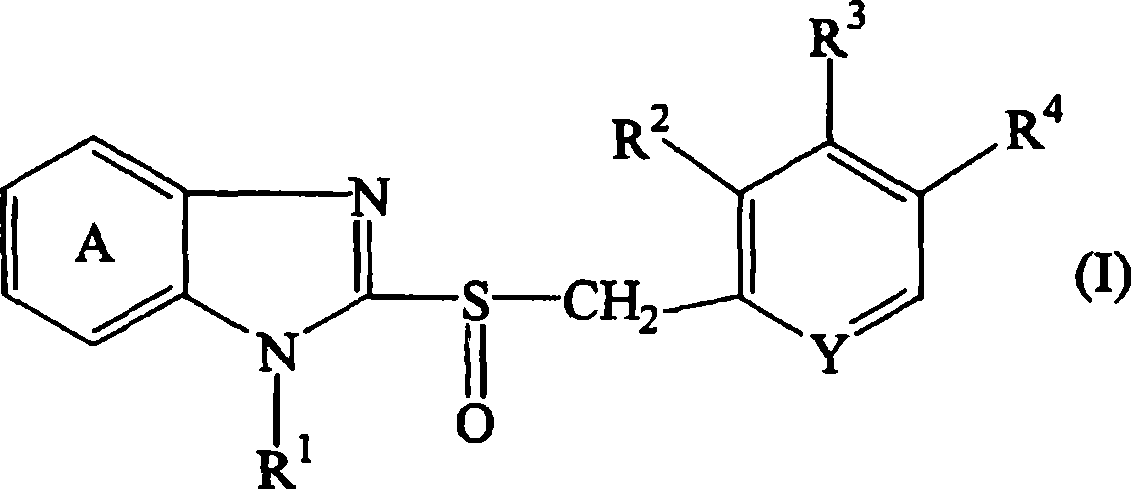
Controlled release solid preparation
A technology of solid preparation and controlled release, which is applied in the direction of pill delivery, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve problems such as difficult pharmacological effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0172] Preparation of Immediate Release Partial Granular Powders
[0173] Lansoprazole (hereinafter referred to as Compound A; 10 g), calcium carbonate (166.67 g) and D-mannitol (155.8 g) were loaded in a fluidized bed granulator, and hydroxypropyl cellulose ( 13.87 g) The mixture was granulated while spraying an aqueous solution in purified water (231.11 g), and then the granules were dried to obtain a granulated powder (340 g) for the immediate-release portion.
preparation example 2
[0175] Preparation of granular powders containing antacids
[0176] Magnesium hydroxide (96.67g), magnesium oxide (133.33g), D-mannitol (121.87g) and crospovidone (10.68g) were loaded in a fluidized bed granulator, and hydroxypropyl The mixture was granulated while cellulose (13.42 g) was sprayed in an aqueous solution in purified water (223.67 g), and then the granules were dried to obtain an antacid-containing granulated powder (370 g).
preparation example 3
[0178] Preparation of core particles
[0179] Core granules serving as the core of the sustained-release portion were prepared as follows. Hydroxypropylcellulose (HPC-SL, 50g) was dissolved in purified water (640g), and low-substituted hydroxypropylcellulose (L-HPC-32W, 25g) and magnesium carbonate (50g) were added to the solution and dispersed in it. Compound A (150 g) was uniformly dispersed in the resulting dispersion to obtain a coating solution. Lactose·crystalline cellulose particles (Nonpareil 105, 100 g) were coated with the compound A-containing coating solution (610 g) using a rotary fluidized bed coater (SPIR-A-FLOW, manufactured by Freund Industry Co., Ltd). . The coating conditions are: inlet air temperature: about 60°C, spray air pressure: about 1kgf / cm 2, exhaust pressure gauge: 100, BED pressure: about 250mmHg, rotor speed: about 300rpm, spray rate: about 6g / min, and spray gun position: lower side. After the coating operation was completed, the obtained fi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com