Zaleplon oral-cavity administration system or composition and preparation method thereof
A zaleplon and oral technology, applied in the field of medicine, can solve the problems of not being able to effectively avoid the first-pass effect of the liver, drug absorption speed and bioavailability, so as to avoid the first-pass effect of the liver, avoid the effect of the digestive tract, and fast effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1 zaleplon spray
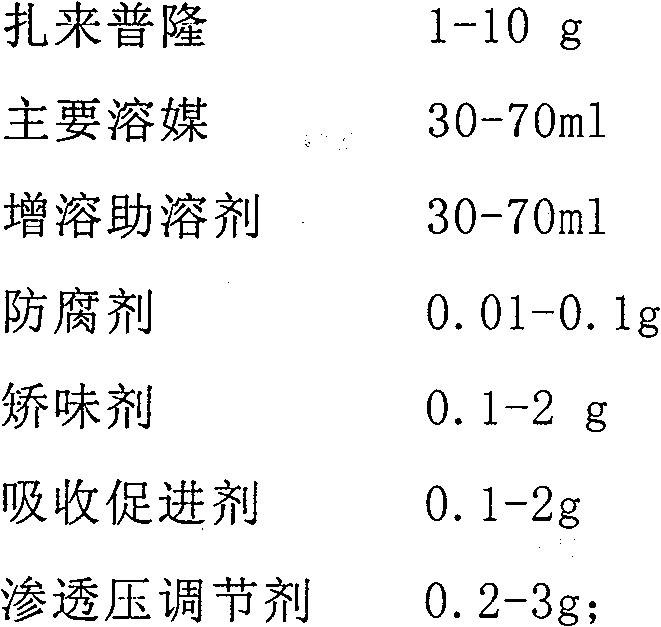
[0038] Prescription ingredient dosage
[0039] Zaleplon 2.5g
[0040] Propylene glycol 50ml
[0041] Ethylparaben 0.05g
[0042] Menthol 0.1g
[0043] Mannitol 0.5g
[0044] Distilled water to 100ml
[0045] Preparation method: Accurately weigh the above-mentioned amount of zaleplon, ethylparaben, menthol, and mannitol, mix well, add the above-mentioned amount of propylene glycol, add water to 100ml, and ultrasonically or vibrate at 40°C until dissolved. The 0.45um microporous filter membrane is used to obtain the Zaleplon sublingual spray solution, which is then dispensed into the spray device.
Embodiment 2
[0046] Embodiment 2 zaleplon spray
[0047] Prescription ingredient dosage
[0048] Zaleplon 2.5g
[0049] Glycerol 50ml
[0050] Ethylparaben 0.05g
[0051] Menthol 0.1g
[0052] Mannitol 0.5g
[0053] Distilled water to 100ml
[0054] Preparation method: Accurately weigh the above-mentioned amount of zaleplon, ethylparaben, menthol, and mannitol, mix well, add the above-mentioned amount of glycerol, add water to 100ml, and ultrasonically or vibrate at 40°C until dissolved , Pass through a 0.45um microporous membrane to obtain zaleplon sublingual spray liquid, and then pack it into a spray device.
Embodiment 3
[0055] Example 3 Zaleplon spray
[0056] Prescription ingredient dosage
[0057] Zaleplon 2.5g
[0058] Macrogol 400 50ml
[0059] Ethylparaben 0.05g
[0060] Menthol 0.1g
[0061] Sorbitol 0.5g
[0062] Distilled water to 100ml
[0063] Preparation method: Accurately weigh the above-mentioned amount of zaleplon, ethylparaben, menthol, and sorbitol, mix well, add the above-mentioned amount of polyethylene glycol 400, add water to 100ml, and ultrasonically or vibrate at 40°C Until it dissolves, pass through a 0.45um microporous membrane to obtain the zaleplon sublingual spray liquid, and then pack it into a spray device.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com