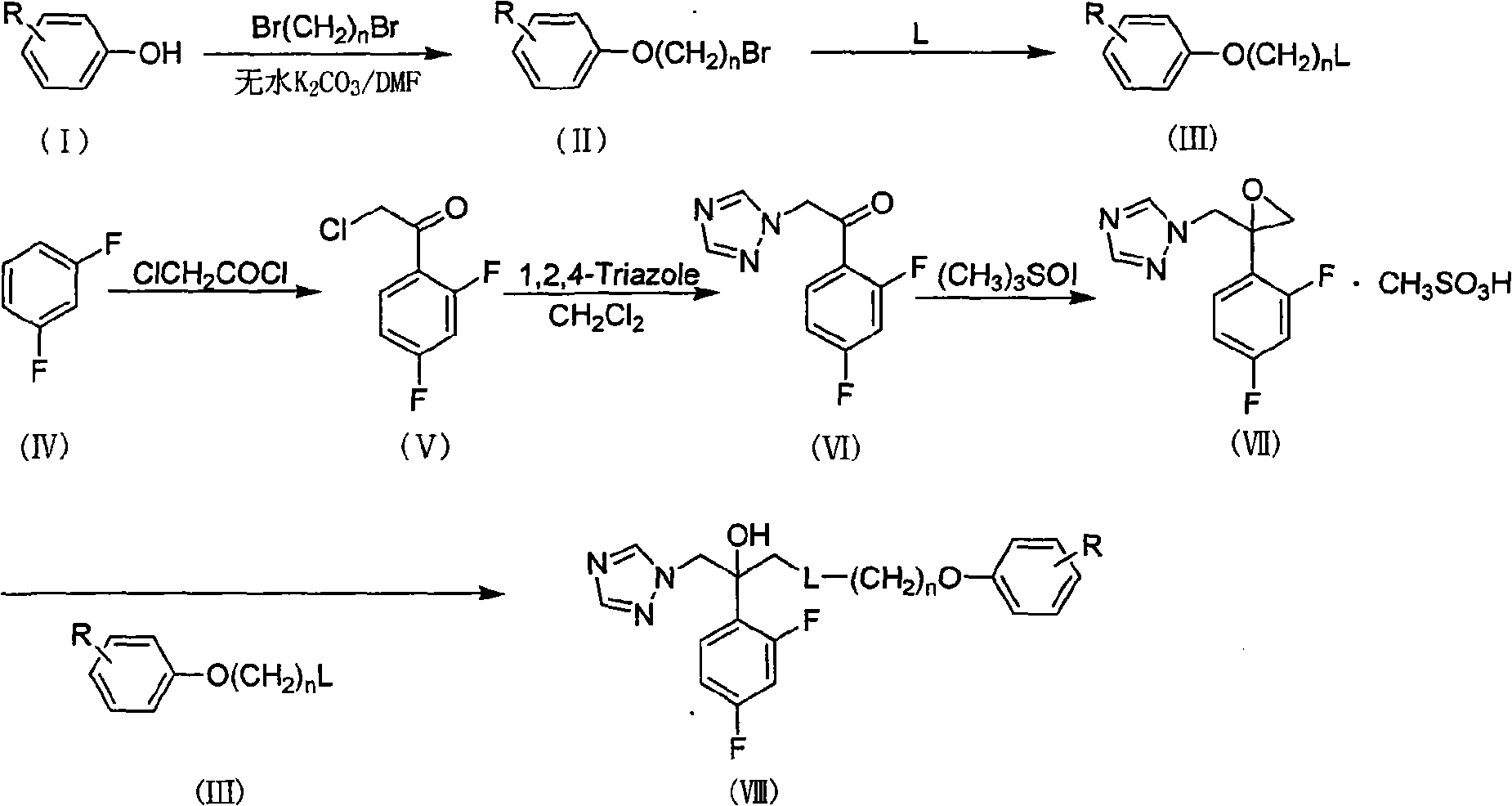
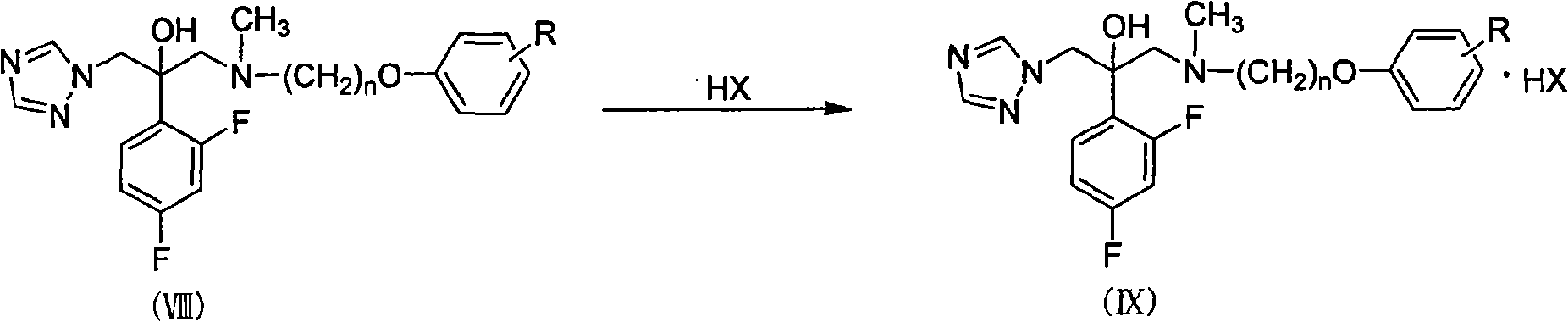
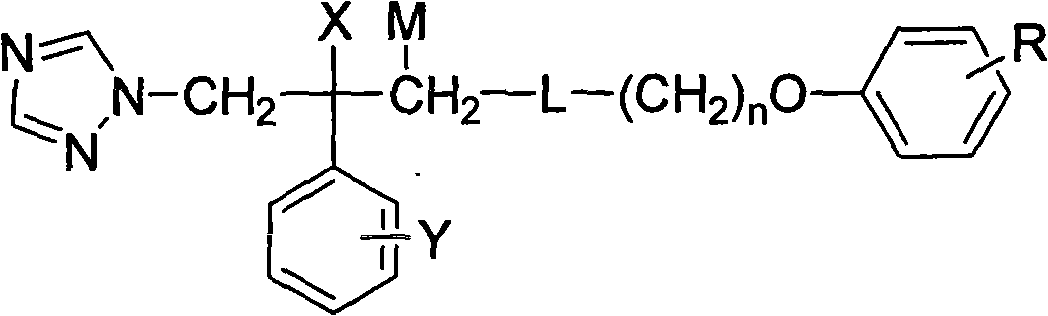Substituted phenoxy oxygen alkylamine triazole alcohol antimycotic compounds and method of preparing the same
A technology of phenoxyalkylamine triazole alcohol and compound, which is applied in antifungal agents, organic chemistry, and pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Embodiment 1: the preparation of bromobutylphenol ether (II)
[0109] Add 43.18g (0.20mol) of 1,4-dibromobutane, 20.73g (0.15mol) of anhydrous potassium carbonate and 100mL of DMF into a 250mL three-necked flask, and add 50mL of DMF solution containing 9.41g of phenol dropwise , after stirring at room temperature for 2 h, the temperature was raised to 70° C., and the stirring reaction was continued for 3 h. After the reaction, filter, dilute the filtrate with 200 mL of ethyl acetate, wash with water (100 mL×3), dry the organic layer with anhydrous sodium sulfate, filter, evaporate the solvent to dryness, and purify the residue by column chromatography (developing solvent: petroleum ether) , to obtain 21.54 g of a colorless transparent oil, with a yield of 94.0%.
[0110] Other type II compounds were obtained by reacting differently substituted phenols with 1,n-dibromoalkanes of different lengths, and repeating the steps in Example 1.
Embodiment 2
[0111] Embodiment 2: the preparation of N-methyl-N-phenoxybutylamine (III)
[0112] In a 100mL round-bottomed flask, add 50mL of methylamino alcohol solution, dropwise add 20mL of ethanol solution containing 5.73g (0.025mol) of bromobutylphenol ether (II) to it, stir and react at room temperature for 12h, and the reaction is almost complete . After the reaction, the solvent was evaporated to dryness to obtain a white solid, which was used in the next reaction without purification.
[0113] Other III compounds were prepared by repeating the steps in Example 2 by performing substitution reactions with different bromoalkyl substituted phenol ethers (II) and methanol solution.
Embodiment 3
[0114] Embodiment 3: the preparation of N-phenoxypropyl piperazine (III)
[0115] In a 50ml round bottom flask, add 20ml of ethanol, 2.94g (0.011mol) of anhydrous piperazine, 2.08g (0.015mol) of anhydrous potassium carbonate, and dropwise add 2.94 pieces of 4-bromopropylphenol ether into it under stirring. (0.01mol) ethanol solution in 20ml, react at 70°C for 4h. After the reaction, evaporate the solvent to dryness, dissolve the obtained solid with 20ml of ethyl acetate, wash with 20ml*3 water, dry the organic layer, filter, and evaporate the solvent to obtain 1.95g of light yellow solid with a yield of 65.22%. Can be used for the next reaction.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com