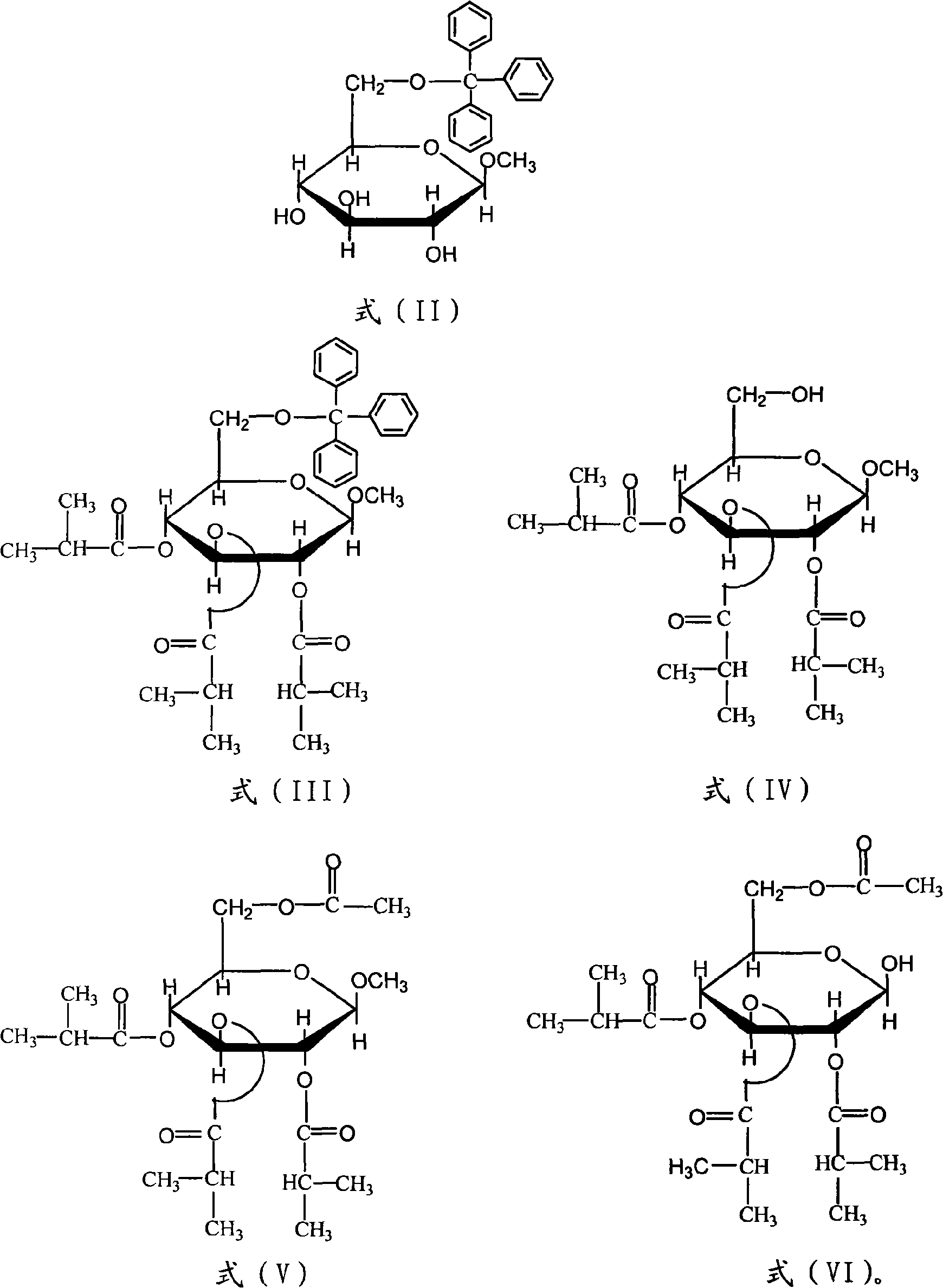Synthesis method for glucose tetra-ester in tobacco
A synthesis method and glucose technology are applied in the field of tobacco flavors and achieve the effects of remarkable industrial application value, easy operation and obvious effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
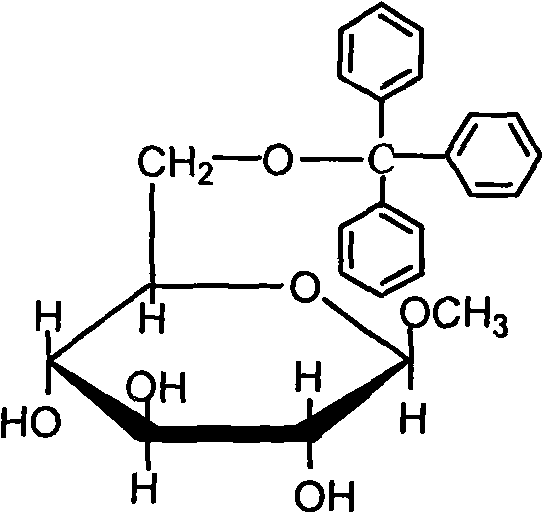
[0032] (1) 6-O-trityl-β-D-methylglucopyranoside (compound of formula (II))
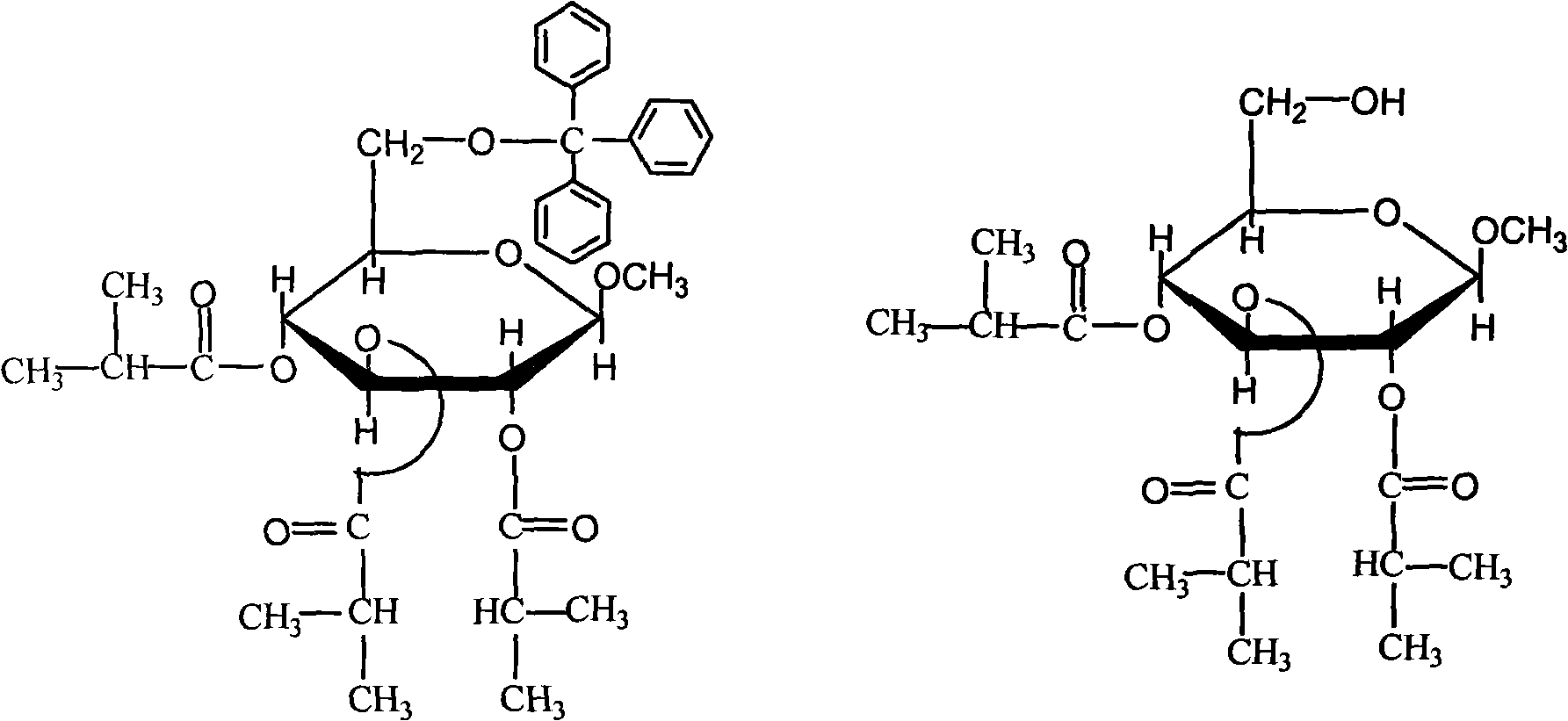
[0033] Add 10.0g β-D-methylglucopyranoside (compound of formula (I)), 16.0g triphenylchloromethane and 100mL pyridine into a 250mL three-necked flask, stir at 100°C for 1h, and pour the reaction solution into In 200mL ice water, extract 3 times with 50mL dichloromethane each time, combine the extracts and wash 2 times with saturated ammonium chloride solution, each time 50mL, then wash several times with ice water, dry over anhydrous sodium sulfate, filter, The syrupy object after removal of the solvent was recrystallized with absolute ethanol to obtain 14.6 g of product with a yield of 65% and a melting point of 155-156° C. (the thermometer was calibrated); it was confirmed to be the target product by infrared and nuclear magnetic resonance analysis. (2) 2,3,4-triisobutyric acid-6-O-trityl-β-D-methylglucopyranoside (compound of formula (III))
[0034] In a 100mL three-necked flask equipped with a ma...
Embodiment 2
[0042] In step (1), control the ratio of β-D-methylglucopyranoside and triphenylchloromethane to 1:1.1, add 30 mL of pyridine, and stir at 100° C. for 1 h. Others are the same as step (1) of Example 1, and the yield of the compound of formula (II) is 65.10%.
[0043] Subsequent steps are the same as in Example 1.
Embodiment 3
[0045] In step (1), control the ratio of β-D-methylglucopyranoside to triphenylchloromethane as 1:1.2, add 25 mL of pyridine, and stir at 100° C. for 1.5 h. Others are the same as step (1) of Example 1, and the yield of the compound of formula (II) is 65.43%.
[0046] Subsequent steps are the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com