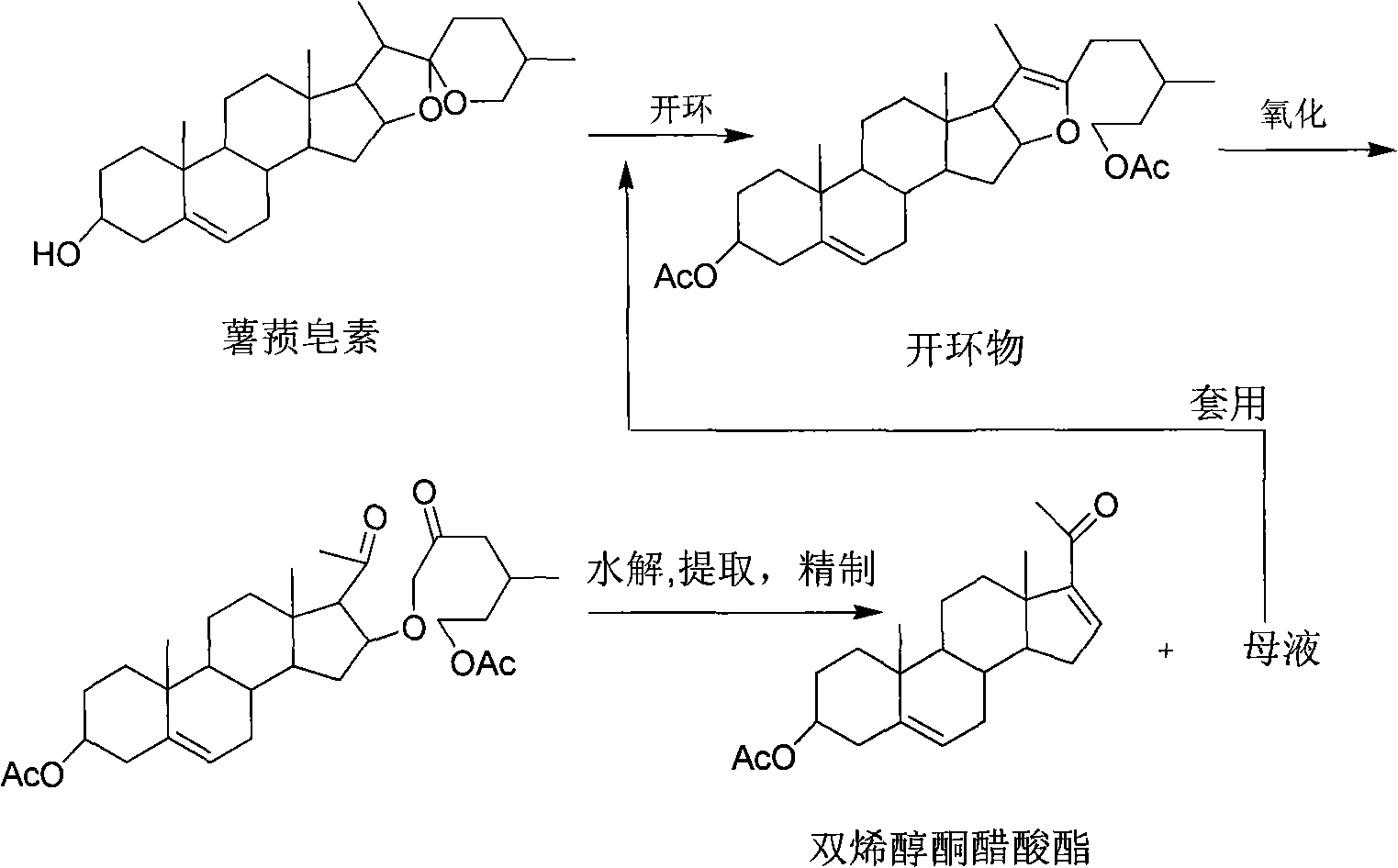
Method for producing high purity diene alcohol ketone acetic ester
A dienolone acetate, high-purity technology, applied in the direction of steroids, organic chemistry, etc., can solve the problem of low quality of dienolone acetate, increased difficulty and instability, reduced yield and product quality, etc. problems, to achieve the effect of low equipment requirements, easy operation, and improved production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Take 1Kg of diosgenin (melting point above 195°C), crack under pressure, oxidize, and hydrolyze (eliminate), extract the hydrolyzate with cyclohexane, remove the acid water from the cyclohexane extract, wash with water until neutral, and Sedimentation and water separation for 24h. Dilute the above-mentioned cyclohexane to a diene concentration of 2%, wet-pack the silica gel column with cyclohexane, and elute with cyclohexane: ethyl acetate = 20:1, and elute to 2BV. This section is an impurity, mainly Contain discarded. Then elute 4BV, stop collecting, combine this section, concentrate to a small volume, and dry by suction filtration to obtain pure white needle-like crystals with a melting point above 169°C and 650g of dienolone acetate fine product with a content of more than 99%. The rate is 65%. The adsorption layer of the silica gel column is dug out, extracted with methanol, and the concentrated extract is recycled and put into the open-loop kettle.
Embodiment 2
[0025] Get dienolone acetate crude product 10g (more than 165 ℃ of fusing points, content 98%), carry out dissolving with normal hexane to concentration containing diene concentration at 1%, with normal hexane wet packing silica gel column, with normal hexane: chloroform = 9:1 for elution, elution to 3BV, this section is a low polarity impurity. Then elute 5BV, stop collecting, combine this section, concentrate to a small volume, and dry by suction filtration to obtain pure white needle-like crystals, 9.6g of dienolone acetate refined product with a melting point above 169°C and a content of more than 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com