Syringe-shaped microorganism culture device
A technology of microorganisms and target microorganisms, which is applied in the fields of enzymology/microbiology equipment, biochemical cleaning equipment, microorganism measurement/inspection, etc., can solve the problems of frequent food poisoning, reduce the complexity of preparation and operation, and avoid complications operation and the effect of shortening the inspection time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
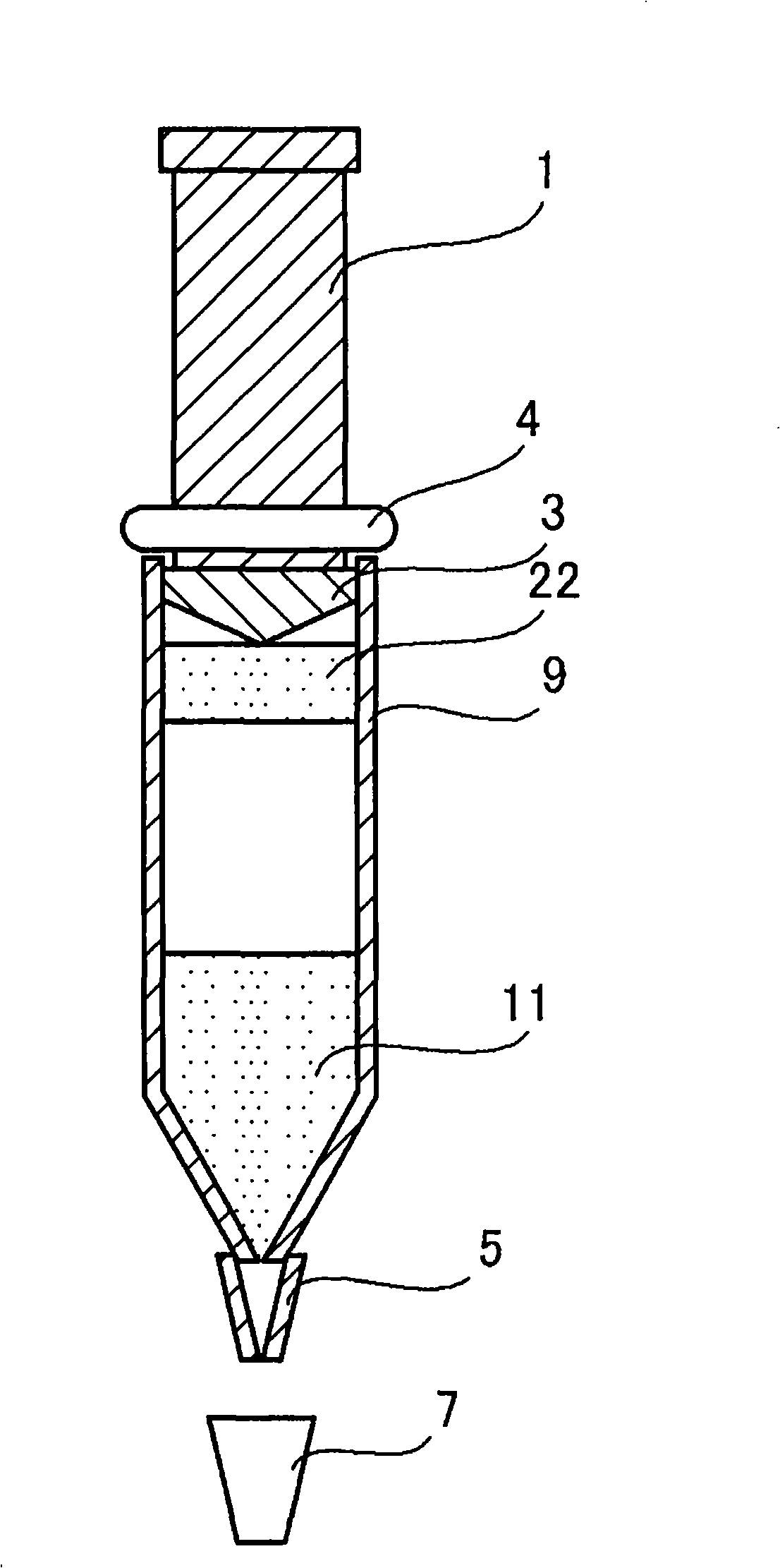
[0049] figure 1 A schematic diagram showing a typical device of the present invention. This device has the shape and structure of a commercially available Luer lock syringe (ルアロッツクテリンジ), and has a cylindrical main body 9 with a tapered front end, a plunger rod 1 slidably inserted into the main body 9, and a taper. The cap body 7 that is buckled (fastened) at the end of the shape. A sheet (or needle) 5 is attached to the tapered end, and the cap body 7 may be configured to cover the sheet (or needle) 5 . At the distal end of the plunger rod, there is a plunger 3 which provides liquid-tight contact with the inner wall of the above-mentioned cylindrical body 9 and thereby prevents the contents contained in the above-mentioned cylindrical body from leaking out. On the bottom of the cap body 7, a silicon rubber layer or the like may be laid to prevent the built-in contents of the above-mentioned main body from leaking from the tapered end or the end of the sheet. A stopper (or O...
Embodiment approach 2
[0054] Fig. 5 shows an overview of a detection method for detecting target microorganisms using the device of the present invention.
[0055] First, 25 g of a sample (sample) was added to 225 mL of buffered peptone water (BPW), and treated with a Stomacher (Stomacher) beating homogenizer to pulverize it. The resulting sample mixture was incubated at 35°C for about 22 hours (pre-enrichment culture). Aspirate a part of the obtained pre-enriched culture solution into a syringe with a capacity of 1.0 mL to 2.0 mL, and fill the syringe with 0.2 ml of the first medium and 0.2 ml of the second medium, wherein the RV medium is used as the second medium. 1 medium, MLCB medium was used as the 2nd medium. At this time, the aspirated pre-enrichment culture solution and the first medium and the second medium are mixed by contact.
[0056] The syringe was erected on a test tube rack, and cultured at 35° C. for about 22 hours (pre-enrichment culture and isolation culture). Presence of tar...
Embodiment 1
[0070] The device of the present invention was produced using a TERUMO syringe (for tuberculin with a capacity of 1 ml: SS-01T) and a TERUMO injection needle (25G x 1 inch) purchased from TERUMO Co., Ltd. (Tokyo).
[0071]Rappapore-Vassiliadis medium (RV medium) (manufactured by MERCK Co., Ltd.) was heated and dissolved in purified water at 1.5 times the normal concentration (62.7 g / l), and glycerol was added to make the concentration 0.5% (w / v) , mixed well and used as enrichment medium. Then, the usual amount (49 g / l) of MLCB agar medium (manufactured by Nissui Pharmaceutical Co., Ltd.) was heated and dissolved in purified water, and glycerin was added to make the concentration 0.5% (w / v), and mixed well , used as the isolation medium. Each medium was sterilized in an autoclave at 115°C for 15 minutes.
[0072] First, about 0.2 ml of the sterilized separation medium described above was aspirated by pulling the plunger rod of the syringe under aseptic conditions. Then, 0.2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com