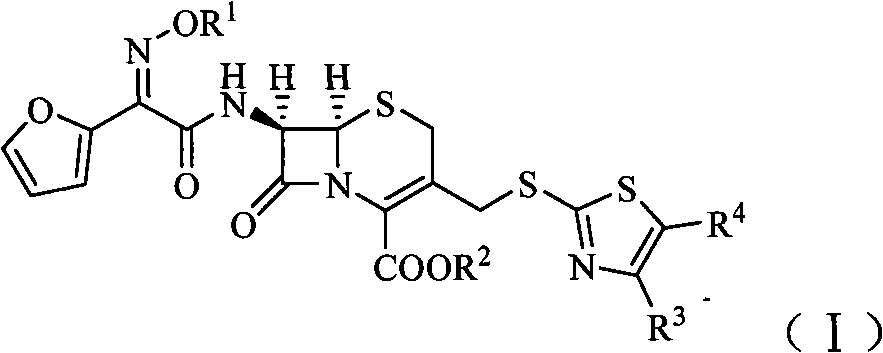
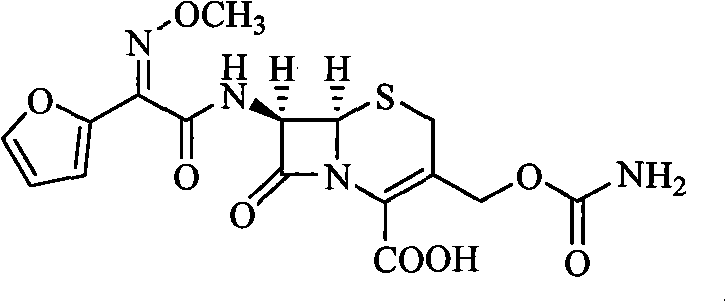
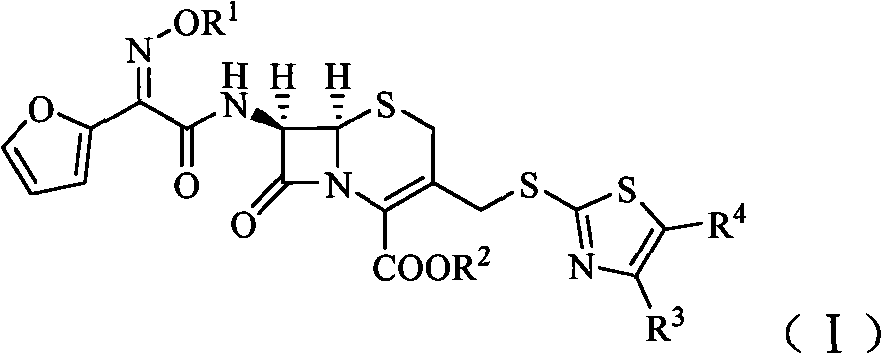
Cephalosporin derivatives
A technology of compounds and halogen atoms, applied in the field of medicine, can solve problems such as affecting the antibacterial efficacy of cephalosporin antibiotics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] Embodiment 1 Compound A, the preparation of compound B
[0115] (1) (6R, 7R)-7-[2-furyl (cis-methoxyimino)acetamido]-3-[[(5-carboxymethyl-4-methyl-2-thiazolyl)sulfur Preparation of ]methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (Compound A)
[0116] Add cefuroxime sodium 1.3g (0.003mol), MMTA 0.6g (0.0032mol), NaHCO 3 0.32g (0.0038mol), 15ml of water, stirred to form a solution, heated in a water bath, and reacted at 60°C for 8h. After the reaction, add a small amount of activated carbon, stir for about 15 min, filter, add hydrochloric acid dropwise to the filtrate under cooling in an ice-water bath until the pH is about 6, then extract 3 times with 20 ml of ethyl acetate (60 ml in total), and the water layer is decompressed to remove residual Ethyl acetate, and then adjust the pH to about 2 with 2N hydrochloric acid, and filter at low temperature for 1 h to obtain 0.9 g of compound A as a yellow solid, with a yield of 54.3%.
[0117] Molecula...
Embodiment 2
[0129] Embodiment 2 Preparation two of compound A and compound B
[0130] (1) Preparation of intermediate TACS (7-amino-3-(5-carboxymethyl-4-methyl-1,3-thiazole-2-mercaptomethyl)ceph-2-ene-2-carboxylic acid)
[0131] Add 2.8g (0.01mol) of 7-ACA and 15ml of water into the Erlenmeyer flask, oscillate to make a uniform suspension for use. Add 2.9g (0.015mol) of MMTA and 40ml of water into a 250ml three-necked flask, add 0.56g (0.014mol) of NaOH solid in batches under stirring, and then use saturated NaHCO 3 Adjust the pH of the solution to 6.0, control the temperature in a water bath at 70°C, then add 7-ACA suspension dropwise and add saturated NaHCO dropwise 3 Solution The pH of the reaction solution was about 6.0, the addition was completed in 15 minutes, and the reaction was performed for 1 hour after the addition. Then add activated carbon and stir for 10 minutes, then filter while it is hot, adjust the pH of the filtrate to 2 with 2N HCl in an ice-water bath, and filter ...
Embodiment 3
[0148] Example 3 Preparation of the compound of the present invention aseptic powder injection
[0149] 1. Prescription:
[0150] Prescription 1:
[0151] Compound B 250g
[0152]
[0153] A total of 1000 sticks were prepared
[0154] Prescription 2:
[0155] Compound B 500g
[0156]
[0157] A total of 1000 sticks were prepared
[0158] Prescription 3:
[0159] Compound B 1000g
[0160]
[0161] A total of 1000 sticks were prepared
[0162] Prescription 4:
[0163] Compound B 2000g
[0164]
[0165] A total of 1000 sticks were prepared
[0166] 2. Preparation process:
[0167] (1) Aseptically process the antibiotic glass bottles, rubber stoppers, etc. used for the preparation;
[0168] (2) Weigh the raw materials according to the prescription, place the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com