Application of 5-(2-methoxy-phenoxy)-2,2'-copyrimidinyl-4,6-diphenol and using method thereof
A bipyrimidinyl and phenoxy technology, applied in the field of 5--2, can solve the problems of high manufacturing cost, inability to meet large-scale digital logic systems, single logic structure, etc., achieve low detection limit, broad application prospects and value , the effect of high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
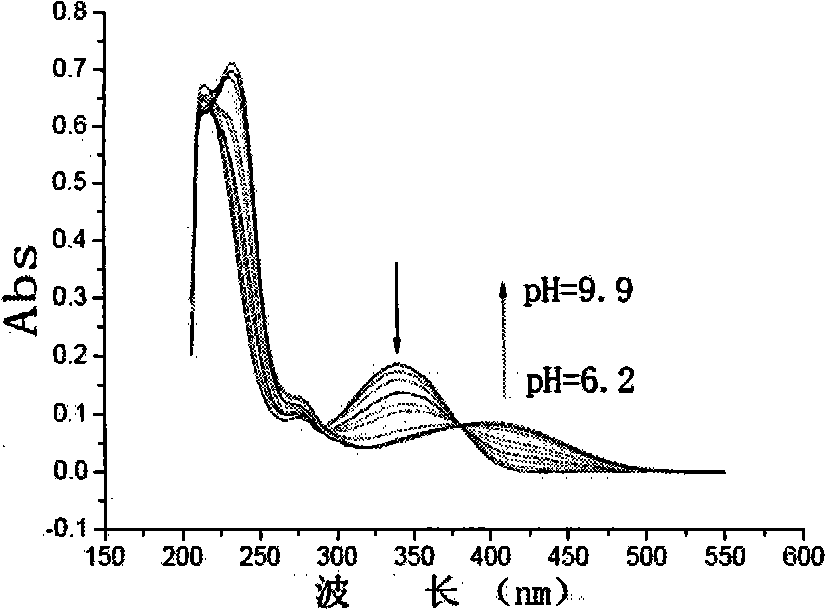
[0029] 1) Dissolve 5-(2-methoxy-phenoxy)-2,2'-bipyrimidinyl-4,6-diol in tetrahydrofuran to prepare a concentration of 2.5×10 -5 mol / L solution, measure the ultraviolet-visible absorption spectrum and fluorescence emission spectrum of the solution, the longest absorption peak of its ultraviolet-visible absorption spectrum is at 340nm, and the fluorescence emission spectrum shows a single peak, the maximum emission wavelength is at 475nm;
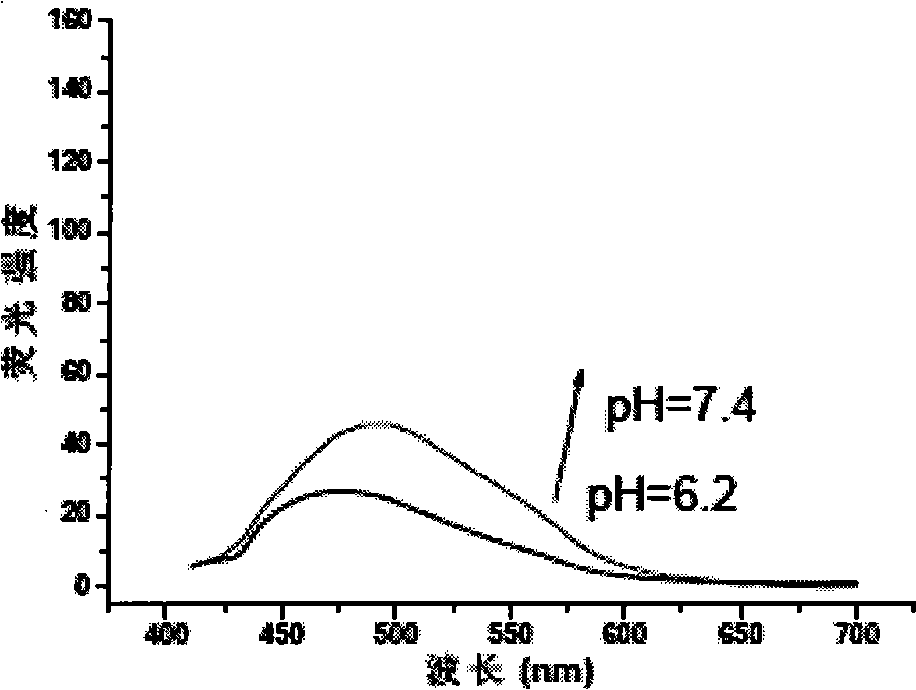
[0030] 2) After adding 0.1 equivalent of tetrabutylammonium hydroxide to the solution, the fluorescence emission peak wavelength red shifts to 490nm, and the intensity is enhanced to twice the original value, and the pH value of the solution is equal to 7.4;
Embodiment 2
[0032] 1) Dissolve 5-(2-methoxy-phenoxy)-2,2'-bipyrimidinyl-4,6-diol in tetrahydrofuran to prepare a concentration of 2.5×10 -5 mol / L solution, measure the ultraviolet-visible absorption spectrum and fluorescence emission spectrum of the solution, the longest absorption peak of its ultraviolet-visible absorption spectrum is at 340nm, and the fluorescence emission spectrum shows a single peak, the maximum emission wavelength is at 475nm;
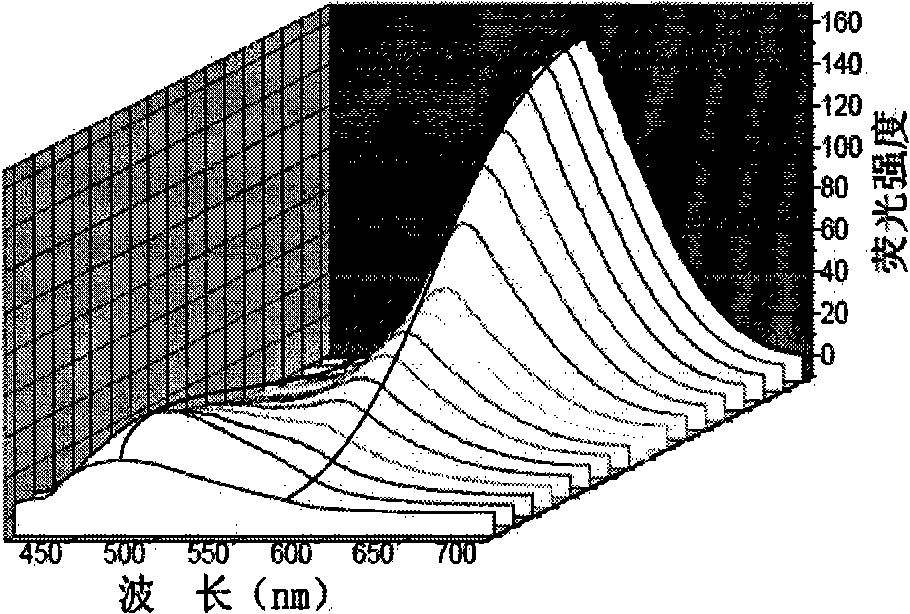
[0033] 2) After adding 6 equivalents of tetrabutylammonium hydroxide in the solution, the fluorescence emission peak redshifted to 565nm, 90nm redshifted than the original emission peak, the fluorescence intensity was 7 times of the original, and the ultraviolet spectrum absorption peak was changed from the original The red shift of 340nm to 410nm, the color of the original colorless solution turns yellow, and the pH value of the solution is greater than or equal to 9.9.
Embodiment 3
[0035] 1) Dissolve 5-(2-methoxy-phenoxy)-2,2'-bipyrimidinyl-4,6-diol in tetrahydrofuran to prepare a concentration of 2.5×10 -5 mol / L solution, measure the ultraviolet-visible absorption spectrum and fluorescence emission spectrum of the solution, the longest absorption peak of its ultraviolet-visible absorption spectrum is at 340nm, and the fluorescence emission spectrum shows a single peak, the maximum emission wavelength is at 475nm;
[0036] 2) After adding 0.1 equivalent of sodium hydroxide to the solution, the fluorescence emission peak wavelength red-shifts to 490nm, and the intensity is enhanced to twice the original value, and the pH value of the solution is equal to 7.4;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com