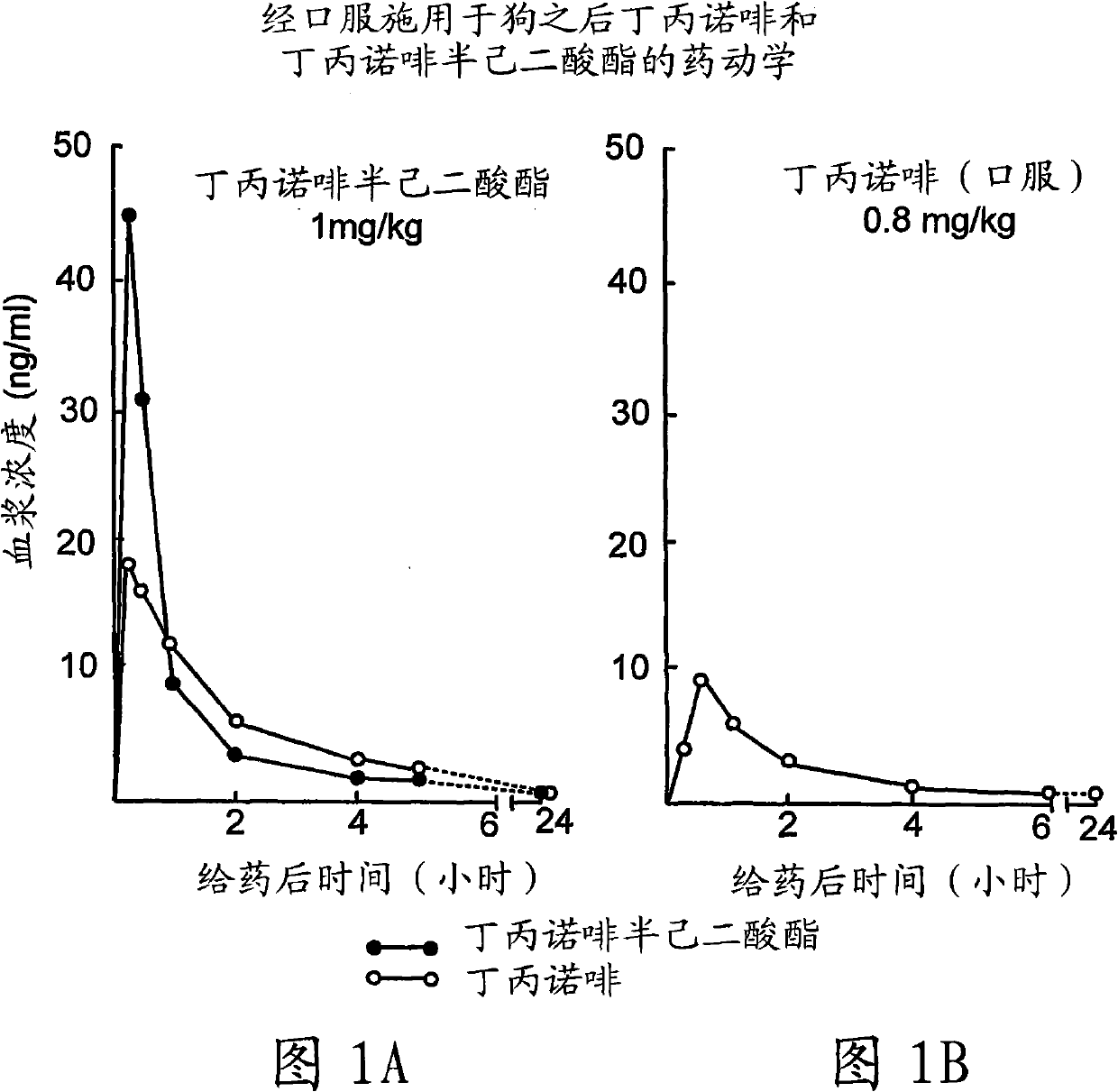
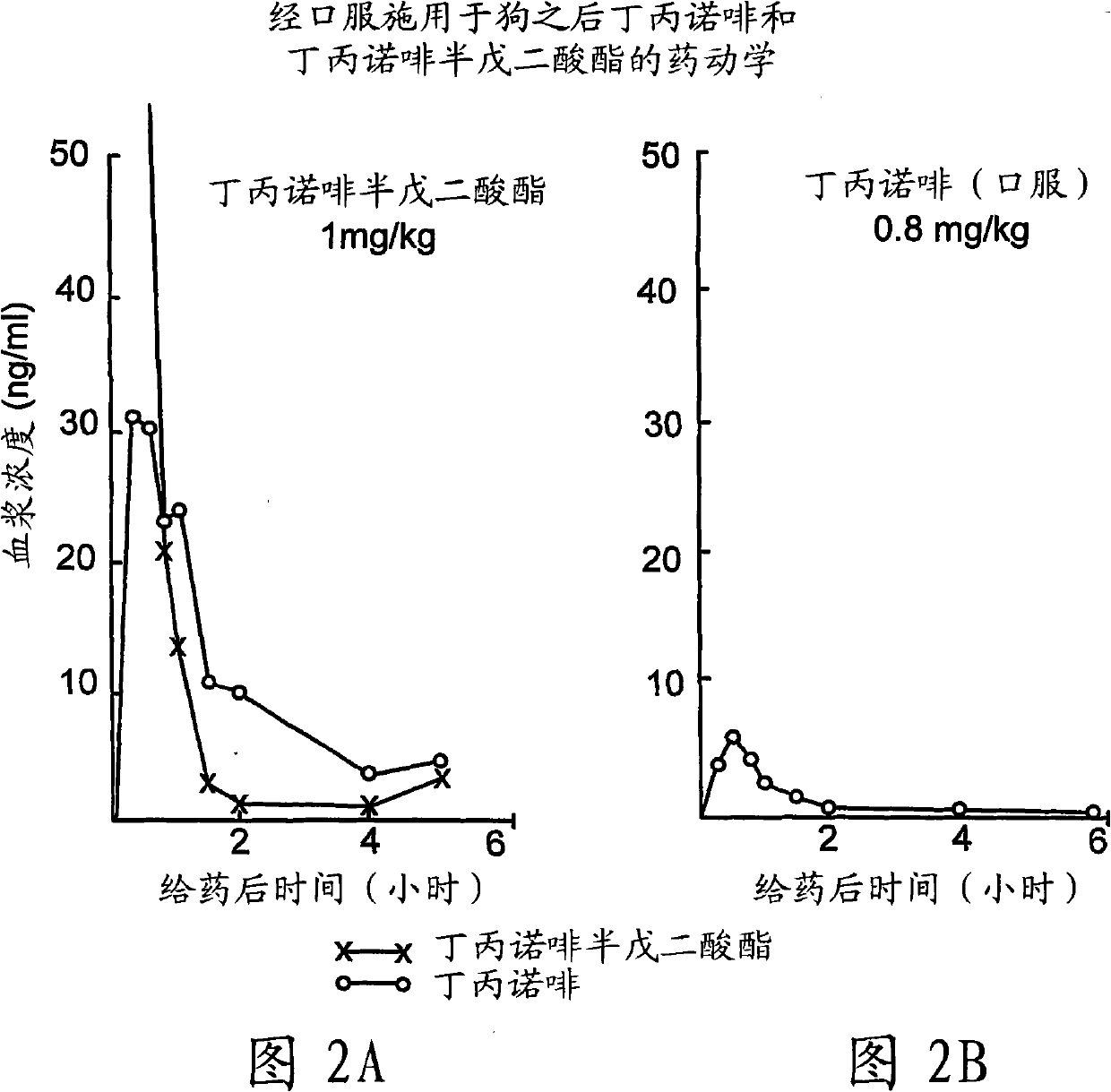
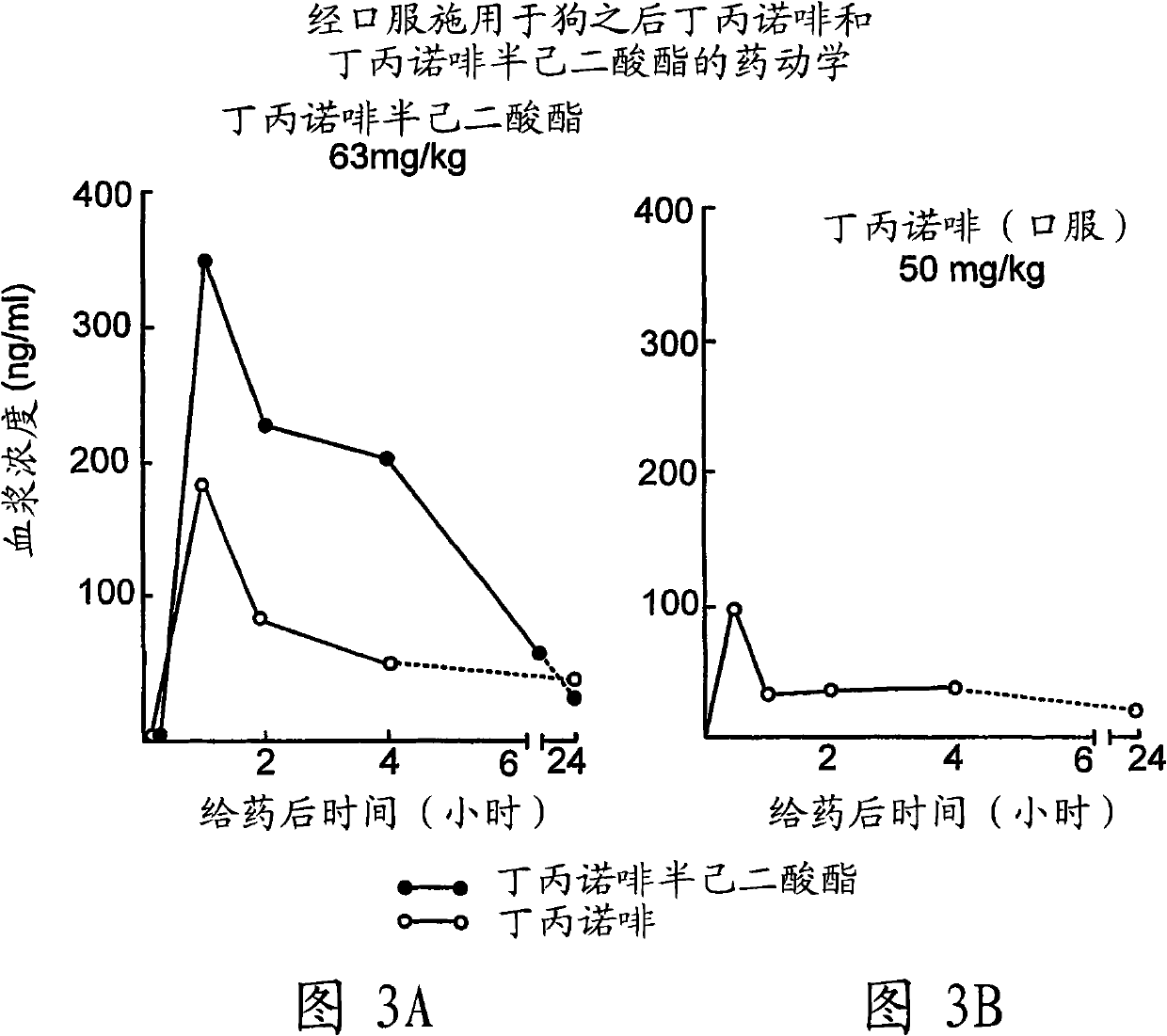
Buprenorphine derivatives and uses thereof
A technology of derivatives and compounds, applied in the field of buprenorphine derivatives, can solve problems such as low bioavailability of buprenorphine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
[0105] Embodiment A: Synthesis of buprenorphine hemisuccinate
[0106] Sodium phenoxide method:
[0107] Buprenorphine (2.35 g, 0.005 mol) was added to sodium hydride (50% dispersion in oil; 0.24 g, 0.005 mol NaH) in 2:1 ethanol:H 2 in warm solution in O (9ml). After stirring for 30 minutes, the solvent was removed by repeated azeotropy with benzene. Finally the residue is dried in vacuo over phosphorus pentoxide. This crude sodium salt was dissolved in dry benzene (30ml), then succinic anhydride (0.5g, 0.005mol) was added, and the mixture was stirred for 1.5 hours. After removing benzene, the residue was shaken with 2N hydrochloric acid (50ml) for 2 hours. The hydrochloride salt thus obtained was filtered, washed with water and dried. Recrystallization from isopropanol followed by washing the filtered product with hot methanol afforded the pure salt (1.0 g), mp 214°C-216°C (dec.). Measured values (percent): C, 64.95; H, 7.6; N, 2.2; Cl, 6.25. C 33 h 43 NO 7 (HCl...
Embodiment B
[0110] Example B: Synthesis of Buprenorphine Hemiglutarate
[0111] A solution of buprenorphine (2.1 g, 0.0045 mol) and glutaric anhydride (1.6 g, 0.014 mol) in dry ether: acetonitrile (3:5, 50 ml) was stirred at room temperature for 5 days, during which time buprenorphine Phenylhemiglutarate glutarate precipitated as a compact white solid. The solid was filtered off and washed with dry ether (40ml). This washing gave the salt (1.4 g) as a white crystalline solid, mp 160°C - 161.5°C. The salt was dissolved in a minimal amount of cold methanol (12ml), then an excess of dry ether (60ml) was added, followed by ethereal HCl. This resulted in the precipitation of a white solid which was filtered off and washed with dry ether. Recrystallization from methanol / ether gave pure buprenorphine hemiglutarate (0.9 g) as hydrochloric acid monohydrate, mp 214°C-215°C (dec.). Measured value (percentage): C63.85; H7.75; N2.08. C 34 h 47 NO 7 (HCl)(H 2 O) requires C64.18; H7.92; N2.20; ...
Embodiment C
[0112] Example C: Synthesis of Buprenorphine Hemiadipate
[0113] Buprenorphine (96 g, 0.2 mol) was dissolved in freshly dried tetrahydrofuran. To this was added adipic acid (129.2 g, 0.8 mol) and DCCI (100 g, 0.48 mol). The mixture was stirred for 6 days before additional adipic acid (30 g) and DECI (25 g) were added. The reaction mixture was stirred for three days. Thereafter, stirring was stopped and the mixture was left to stand for three days. The solid was filtered off and the solvent was removed from the soluble material. The resulting solid was dissolved in a minimal amount of methanol followed by addition of ethanol / HCl to give buprenorphine hemiadipate hydrochloride as a solid (86 g), which was purified by recrystallization from ethanol. The purified material has a melting point of 270°C to 272°C (decomposition). Measured values (percent): C, 66.49; H, 8.12; N, 2.23; Cl, 5.53. C 35 h 50 NO 7 Cl needs C, 66.49; H, 7.97; N, 2.22; Cl, 5.61; 3440 (OH), 176...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com