5-beta-sapogenin and pseudosapogenin derivatives and their use in the treatment of dementia
A technology of aglycone and derivatives, applied in the field of 5-beta saponin and pseudosaponin derivatives and their use in the preparation of drugs for treating dementia, can solve basic key defects, Lack of synaptic transmission, no supply, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
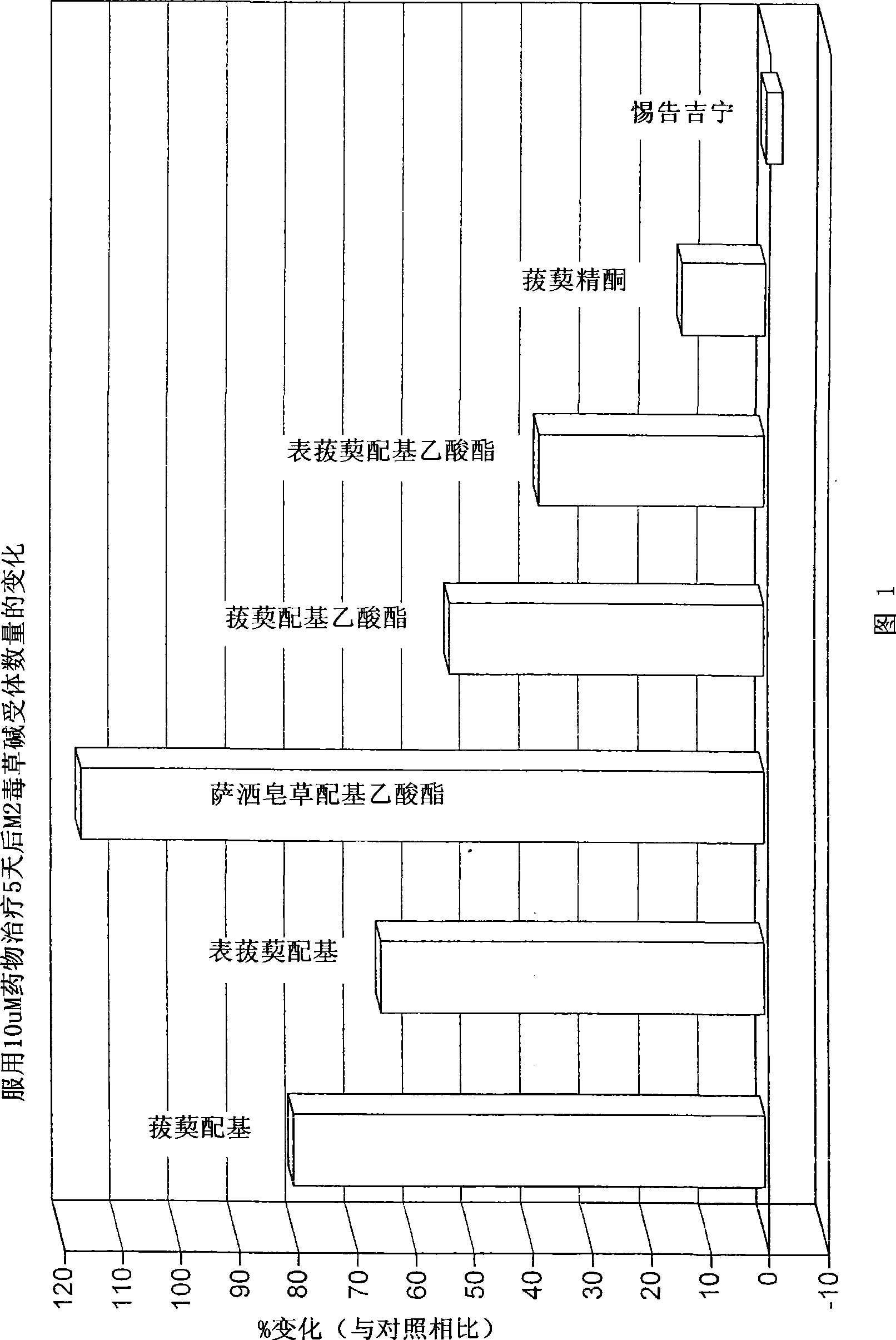
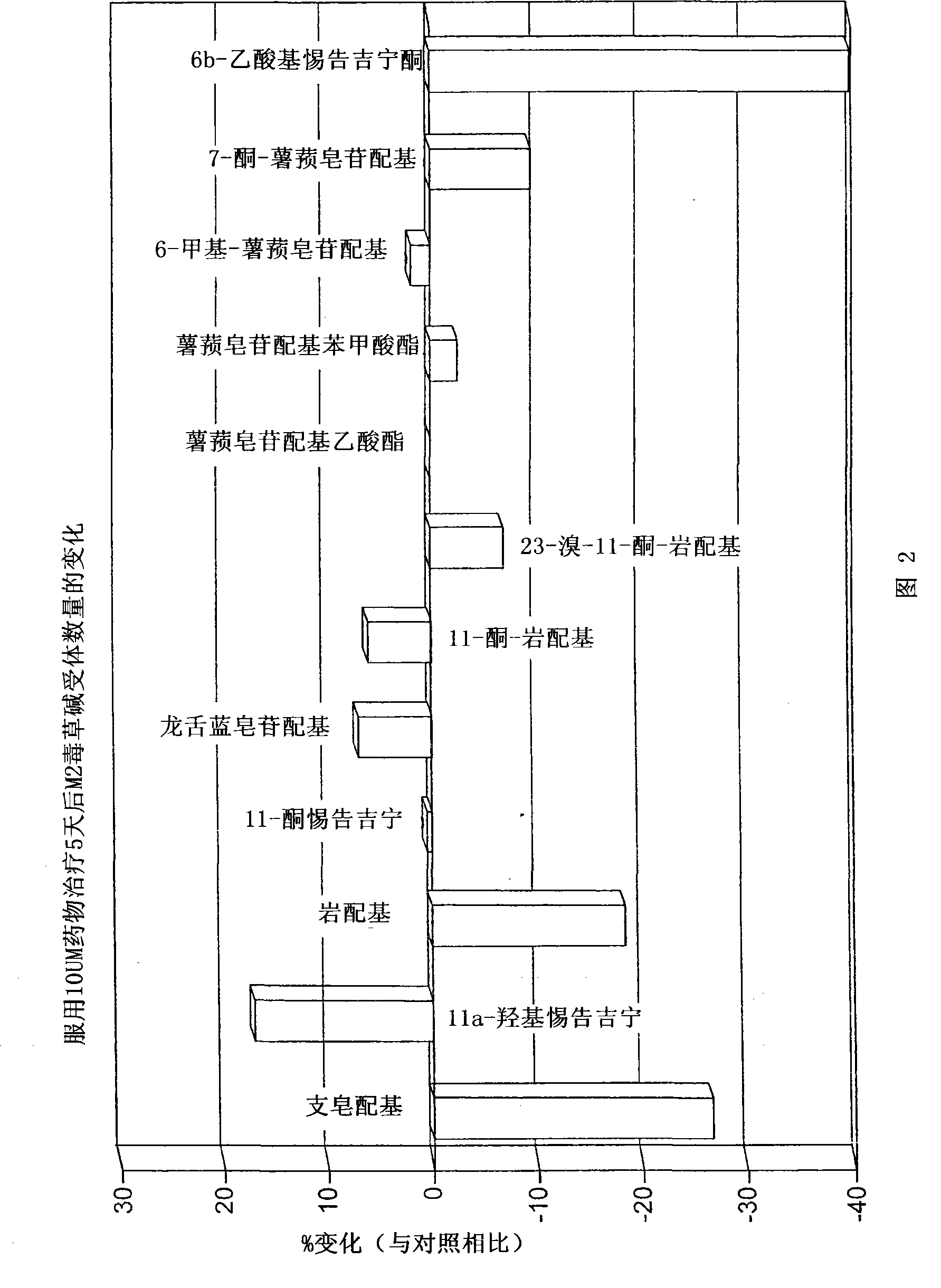
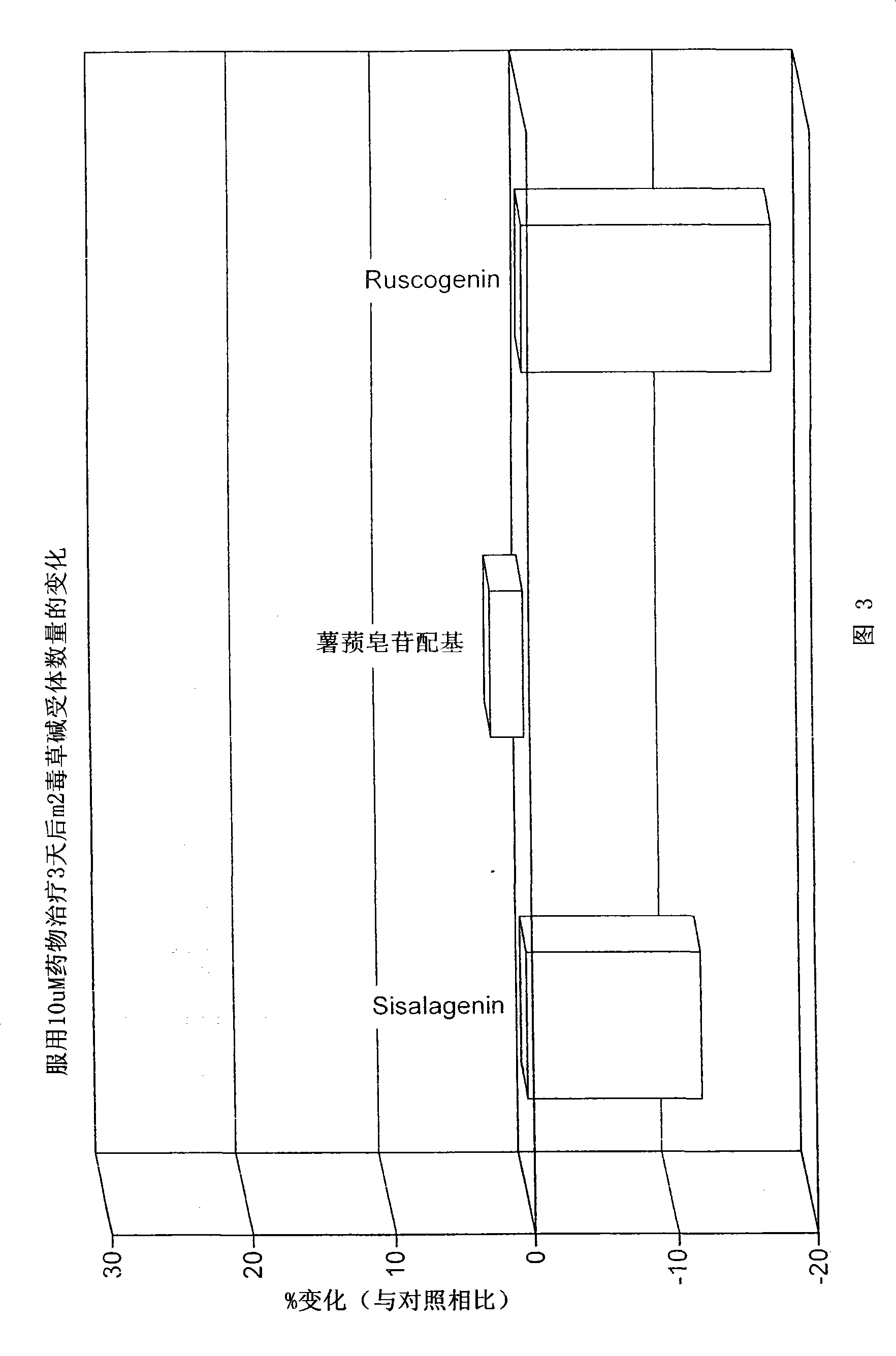
[0151] In CHO cell lines expressing recombinant human muscarinic receptors in vitro, the number of muscarinic receptors tended to decrease over time. Muscarinic receptor density was increased after 72 hours of incubation with saponin derivatives (1-10 μm) according to the invention.
[0152] method:
[0153] Effect of saponin derivatives of the present invention on muscarinic receptor density in CHO cells expressing recombinant human muscarinic receptors.
[0154] Chinese hamster ovary (CHO) cells expressing high levels of receptor (-2.2 pmole receptor / mg protein) were cultured in flasks (150 ml) for 24 hours before starting the experiment. Vehicle (DMSO) and saponin derivatives (1 and 10 [mu]M) were added to the medium for 48 hours. The culture medium was removed, the cells were scraped and resuspended in Hanks solution, centrifuged, and passed through with [ 3 [H]-QNB were incubated for 30 minutes, after which the level of m receptor was determined by liquid scintillation...
Embodiment 2
[0158]
[0159] 3-O-2Oxycarbonyl-5β, 20α, 22α, 25R-spirostan-3β-ol
[0160] Ethyl chloroformate (1.40g, 12.9mmol) was added dropwise to a solution of smilagenin (2.08g, 5.0mmol) in anhydrous dichloromethane (15ml) and anhydrous pyridine (1.02g, 12.9mmol). in the stirred solution. The mixture was stirred at room temperature for 18 hours, then partitioned between water (30 mL) and dichloromethane. The aqueous layer was extracted twice with dichloromethane, and the combined organic layers were washed with water, then dried over magnesium sulfate (anhydrous). The solvent was evaporated in vacuo to give an oil which crystallized rapidly (2.1 g). This material was chromatographed on silica gel (about 70 g), eluting with ethyl acetate-hexane (1:9), and recrystallized from methanol to give 3-O-2oxycarbonyl-5β,20α,22α,25R-spiro White crystals of stan-3β-ol (1.08 g).
[0161] mp154-156℃; m / z 488 (M + for C 30 h 48 o 5 ); 1 H nmr (270MHz, CDCl 3 )δ 0.76 (3H, s, 18-CH 3 ), 0...
Embodiment 3
[0163] Epismilagen succinate
[0164]
[0165] A solution of epismilagenin (200 mg, 0.48 mmol) and succinic anhydride (60 mg, 0.59 mmol) in anhydrous pyridine was stirred overnight at room temperature under nitrogen. Another portion of succinic anhydride (120 mg, 1.18 mmol) was added and the reaction mixture was stirred for an additional 24 hours. After adding another portion of succinic anhydride (120 mg, 1.18 mmol), the reaction mixture was heated at 50° C. for a further 24 hours with stirring. After the reaction was cooled, water (10 mL) was added. The aqueous solution was extracted with ether (4 x 20 mL). The combined organic extracts were washed with water (3 x 20 mL). Dry (anhydrous magnesium sulfate), filter. The solvent was evaporated in vacuo to give an orange oil (1.8 g), which was chromatographed on silica gel using ethyl acetate / petroleum ether (1:4) as eluent. Recrystallization from acetone gave white crystals of epismilagenyl succinate (87 mg).
[0166] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com