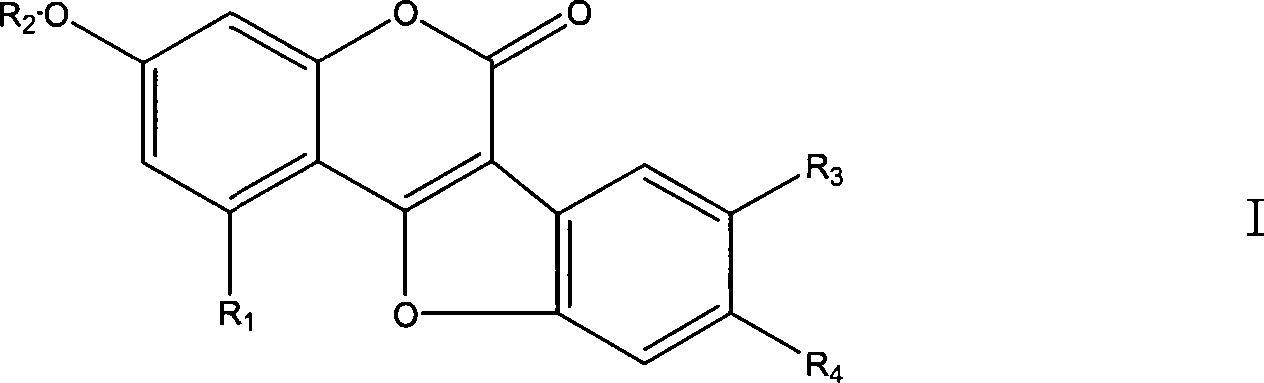
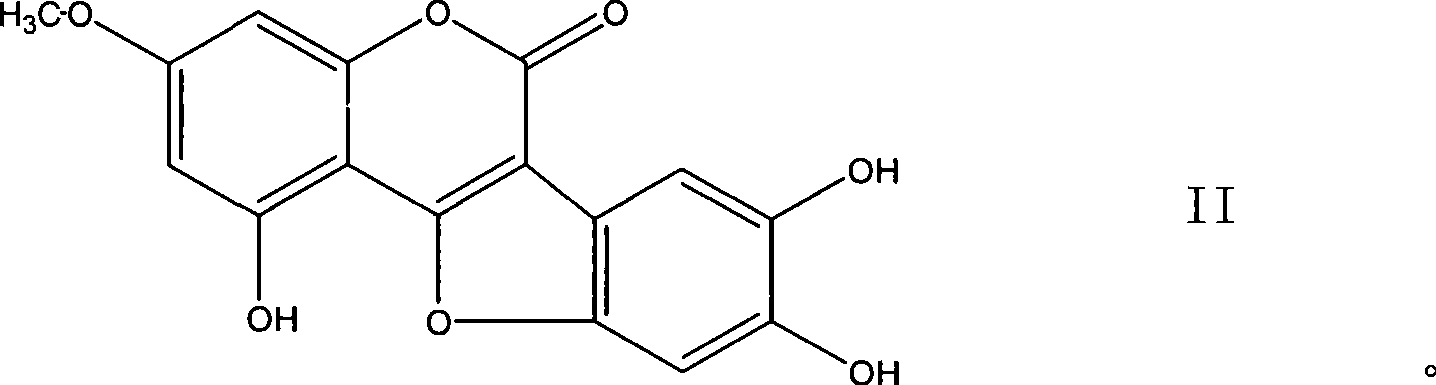
Use of coumarether and composition thereof
A kind of technology of coumarin and compound, applied in the field of pharmacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] The compound wedelolide extracted from Eclipta alba
[0071] (1) soaking and filtering
[0072] Eclipta whole grass 300kg, fully immersed in 0.75 tons of ethanol (concentration 95%)→soak overnight (10 hours)→coarse filtration, thereby removing the whole grass residue (retention)→net filtration (suction filtration) or high-speed centrifugation (10000rpm, 10 minutes), thereby removing dust and fine residue → green clarified filtrate.
[0074] Recover ethanol by distillation, the temperature does not exceed 60°C → distill for 2 hours each time, remove the extract in the reaction kettle to the collection bucket (the extract is dark dark green, slightly viscous) → repeat the above steps until all ethanol is recovered .
[0075] (3) Second soaking and back distillation
[0076] Recover 0.75 tons of ethanol, re-soak the whole herb residue → soak overnight, the requirements for coarse filtration, net filtration and distillation recovery are the...
Embodiment 2
[0089] Therapeutic effect of wedelolide on cyclophosphamide-induced leukopenia in mice
[0090] Experimental animals: 60 male SPF grade C57BL / 6 mice, purchased from Shanghai Slack Experimental Animal Co., Ltd., weighing 22-24 g. Raised in SPF grade animal room, 12h light / 12h dark, free intake of feed and water.
[0091] experimental method:
[0092] 1. Modeling method:
[0093] In addition to the normal control group, the rest of the animals were randomly divided into 5 groups, intraperitoneal injection of cyclophosphamide 100mg / kg for 3 consecutive days to create a leukopenia model;
[0094] 2. Administration situation:
[0095] (1) normal group, (2) model group, mice in (1) (2) group were given normal saline with a volume of 0.1ml / 10g; (3) (4) groups were Li Kejun high and low dose groups : Mice were given doses of 40mg / kg and 10mg / kg of Li Kejun respectively; (5) (6) (7) groups were APL-1 high, middle and low dose groups: mice were given doses of 100mg / kg kg, 25mg / kg, ...
Embodiment 3
[0107] Wedelide pair 60 Protective effect of Co-γ-induced bone marrow injury in mice
[0108] Experimental animals: 60 male SPF grade ICR mice, purchased from Shanghai Slack Experimental Animal Co., Ltd., weighing 22-24 g. Raised in SPF grade animal room, 12h light / 12h dark, free intake of feed and water.
[0109] experimental method:
[0110] 1. Modeling method:
[0111] Except for the animals in the normal control group, the rest of the animals were randomly divided into 5 groups. 60 Co-γ ray was irradiated once with a dose of 7.50Gy (dose rate 450 Rm / min).
[0112] 2. Administration situation:
[0113] (1) normal control group, (2) model group, (1) (2) group mice were given normal saline with a volume of 0.1ml / 10g; Groups: Mice were given 40 mg / kg and 10 mg / kg of Likejun; (5) (6) (7) groups were APL-1 high, medium and low dose groups: Mice were given 100 mg / kg, 25mg / kg, 5mg / kg of APL-1. All the above groups were intragastrically administered 7 days before irradiati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com