Tetrahydro chinolines derivates and synthetic method
A technology of tetrahydroquinolines and derivatives, applied in organic chemistry and other directions, can solve problems such as difficulty, severe exothermic reaction, and decreased functional group compatibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0047] The following examples help to understand the present invention, but do not limit the content of the present invention.
[0048] Embodiment --- the general operating procedure of reaction
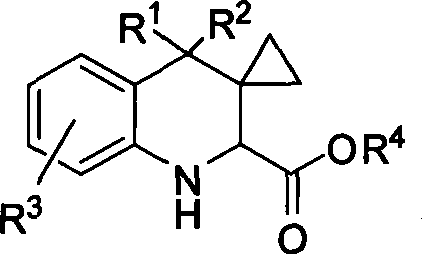
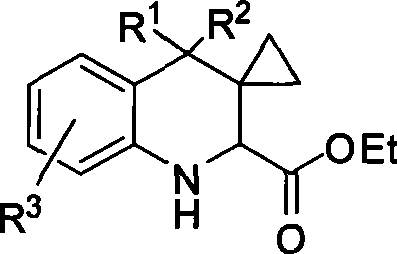
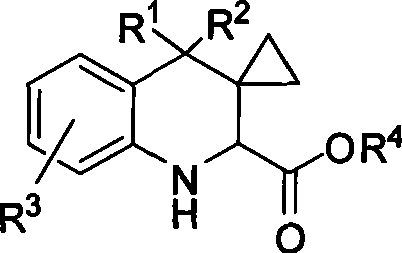
[0049] Synthesis of tetrahydroquinoline derivative 3: In a dry reaction tube, add methylene cyclopropane (1, 0.30 mmol), imine of ethyl glyoxylate (2, 0.3 mmol), montmorillonite K- 10 (50 mg) and DCE (1 mL) were stirred at room temperature for 3 hours. Flash column chromatography (SiO 2 , the eluent is ethyl acetate / petroleum ether=1 / 6) to obtain the corresponding product 3.
[0050] Synthesis of imidazoline derivative 4: In a dry reaction tube, 1M triethylaluminum in toluene (0.6mL, 0.6mmol) was diluted with 1mL of toluene, and 1mL of ethylenediamine (40L, 0.6mmol) was added at 0°C Toluene solution. After stirring at room temperature for 1 h, a solution of 3a (139 mg, 0.3 mmol) in 2 mL of toluene was added. Stir at reflux for 10 hours, cool to 0°C, and quench the reaction with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com