Reversibly modified thermostable enzyme compositions and methods of making and using the same
A thermostable enzyme and thermostable technology, which can be used in biochemical equipment and methods, microbial determination/inspection, enzymes, etc., and can solve the problems of long modification process, cumbersome efficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0186] Preparation of flap endonuclease-1 and Taq DNA polymerase
[0187] The DNA of Archaeoglobus fulgidis was obtained from ATCC (49558D). The gene encoding the flap endonuclease-1 (Afu FEN-1 ) of Archaeoglobulin fulgidis was cloned by PCR as described by Hosfield et al. (Hosfield, 1998, JBC 275(22): 16420-16427). The cloned sequences were verified by direct sequencing. The Afu FEN-1 gene was cloned into pET-28 (Noragen). Overexpression and purification of Afu FEN-1 protein was accomplished according to the method of Hosfield et al. with minor modifications.
[0188] Thermus aquaticus strain YT-1 was obtained from ATCC (25104). The DNA polymerase gene of Thermus aquaticus (Taq) was cloned by PCR using the sequence from GeneBank (Acession No. J04639). Plasmid pET-28 was used to construct the expression vector. Taq DNA polymerization was performed according to the method described by Lawyer et al. (Lawyer et al., 1989, JBC 264(11):6427-37; Lawyer et al.1989, PCR Meth.Ap...
Embodiment 2
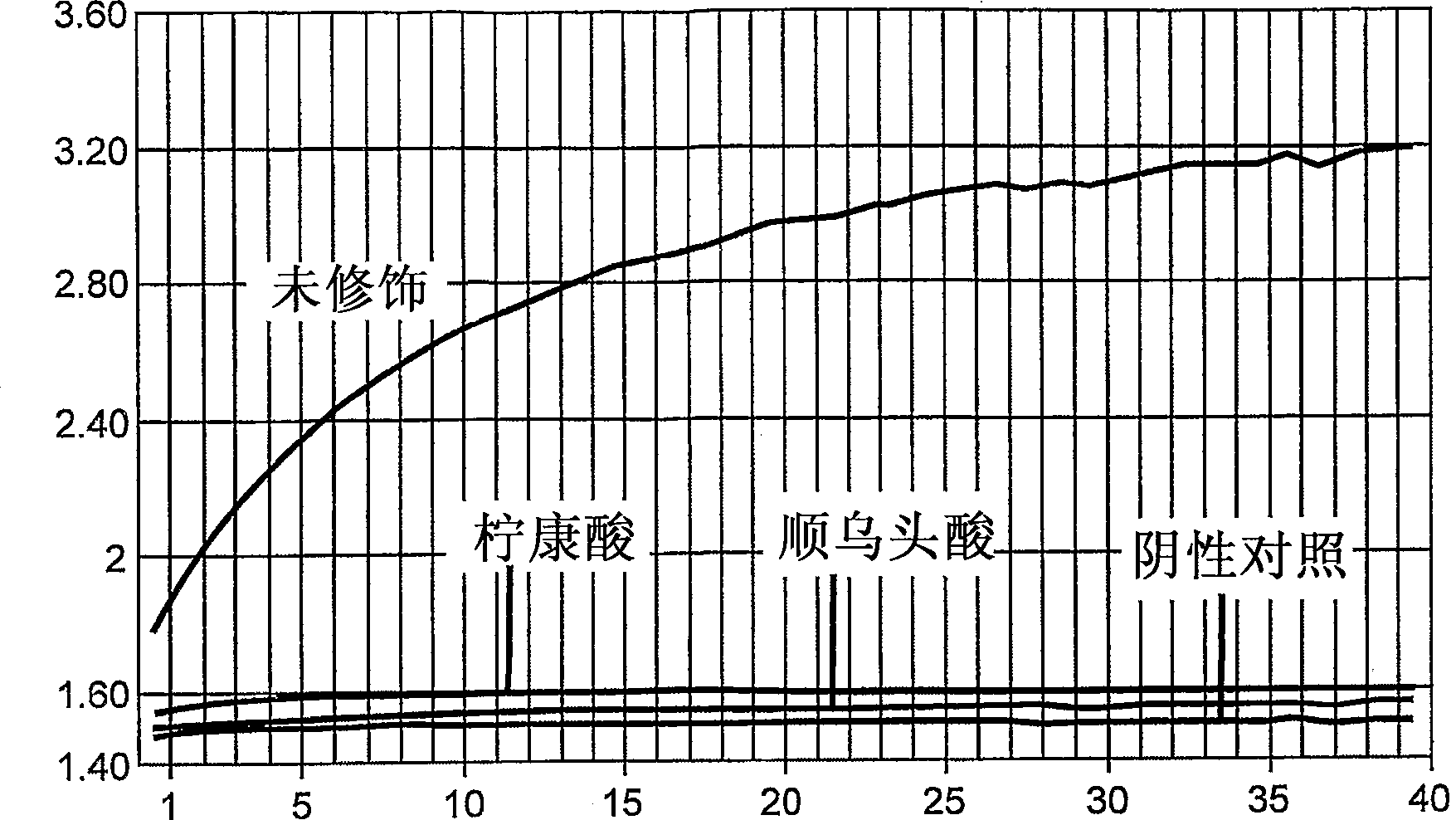
[0190] Modification of Afu FEN-1 with citraconic acid
[0191] Modification of Afu FEN-1 with citraconic acid was performed in a buffer containing 20 mM MOPS, pH 8.0 and 100 mM KCl. The concentration of Afu FEN-1 was adjusted to 1 mg / ml.
[0192] Citraconic acid (Aldrich) and N,N'-dicyclohexylcarbodiimide (DCC) (Aldrich) were dissolved in N,N'-dimethyl-formamide (DMF) (Fisher, sequencing grade), The concentration is 1M. 100 μl of 1M citraconic acid and 200 μl of 1M DCC were mixed in a 1.5 ml Eppendorf tube. The mixture was incubated at room temperature for 1 hour. The mixture was then centrifuged at 12,000 rpm for 20 minutes at room temperature. The precipitate was discarded and the supernatant was retained for modification of Afu FEN-1.
[0193]One volume of activated citraconic acid was mixed with 99 volumes of Afu FEN-1. The mixture was then incubated at room temperature for 1 hour to obtain chemically inactivated Afu FEN-1.
Embodiment 3
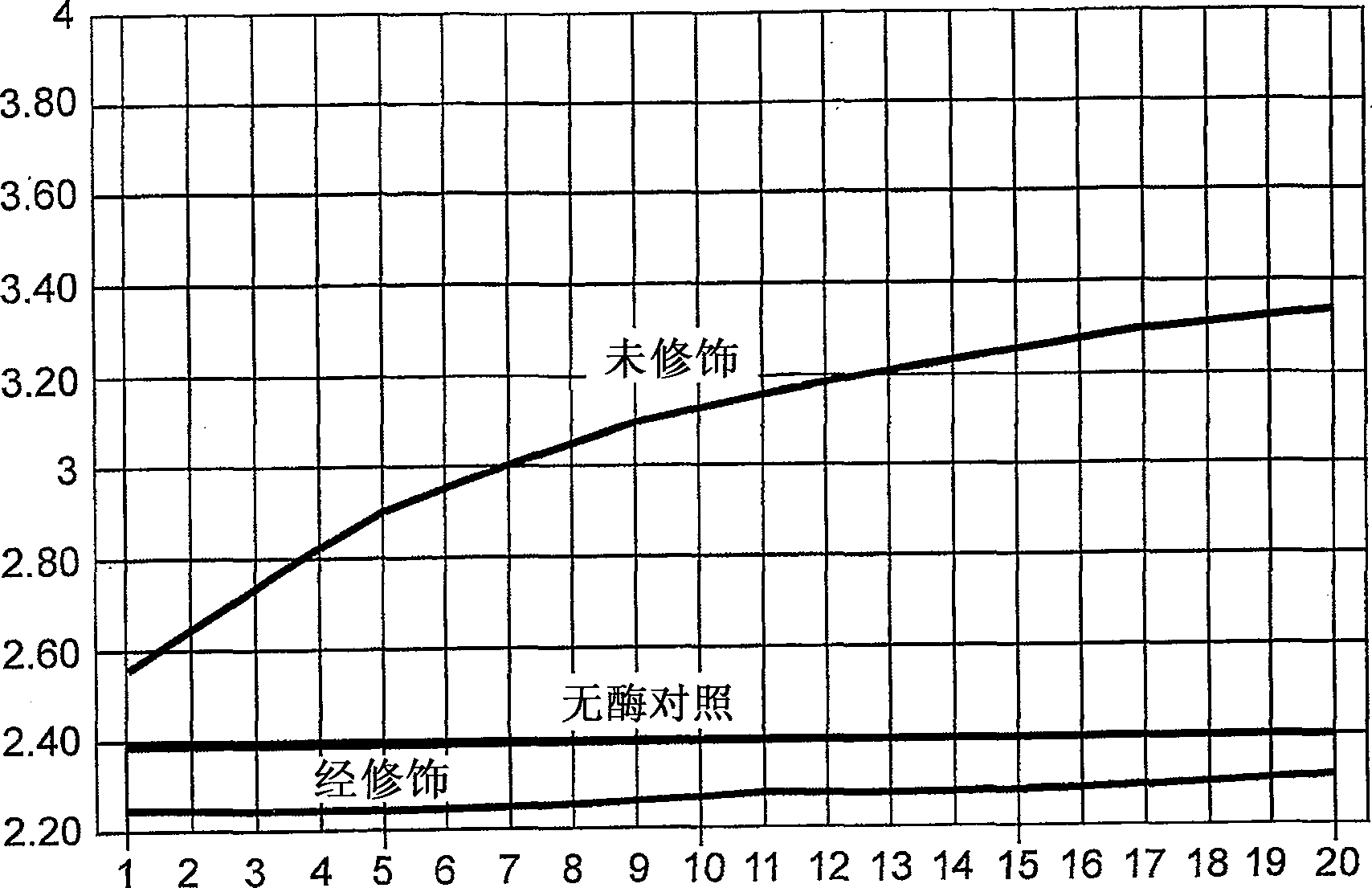
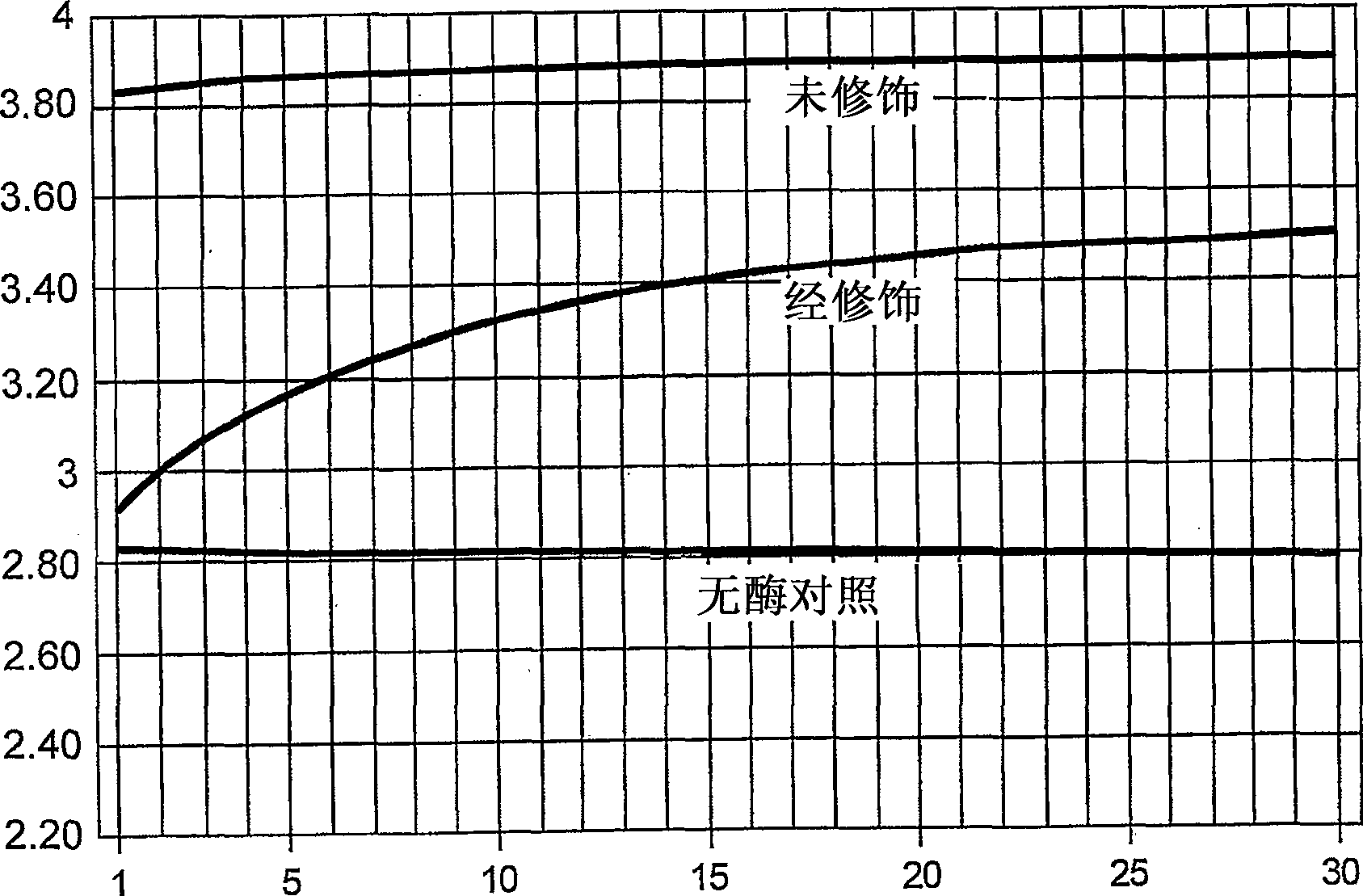
[0195] Activity assay of modified Afu FEN-1
[0196] The flap endonuclease activity of the modified Afu FEN-1 was determined. Control reaction mixture without enzyme contained 30mM Tris HCl pH8.0, 3mM Mg 2+ , 400 nM 5-ROX (Sigma), 0.01% Tween-20, 100 nM each of the following nucleic acids 18SI, 18SP, 18ST (see sequence information in Table 1). Both 18SI and 18SP consist of sequences complementary to 18ST. 18SI is located upstream of 18SP and overlaps with 18SP by 1 nucleotide. When 18SP is intact, the 6Fam fluorescence of 18SP is quenched. In the presence of Afu FEN-1, 18SP in a complex containing 18SI, 18SP and 18ST is cleaved by Afu FEN-1. This cleavage results in an increase in 6FAM fluorescence. Add 10 ng of chemically modified Afu FEN-1 to a 25 μl reaction. The same amount of unmodified enzyme was used as a control.
[0197] Activity assays were performed on an ABI Prism 7000 to detect changes in fluorescence intensity in real time. The incubation conditions wer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com