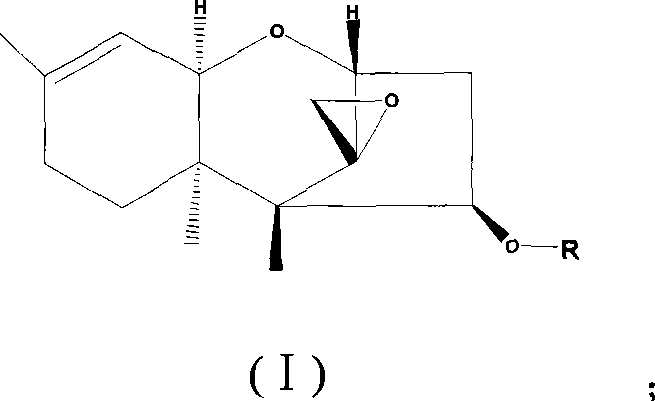
Trichodermin derivant and uses thereof
A technology of trichoderma and derivatives, applied in the directions of biocides, chemicals for biological control, applications, etc., can solve the problems of no synthesis and application related reports, etc., and achieve the effect of good activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1, a kind of synthetic method of trichodermalin derivative a-1, its reaction formula is:
[0053]
[0054] Concrete reaction process is as follows:
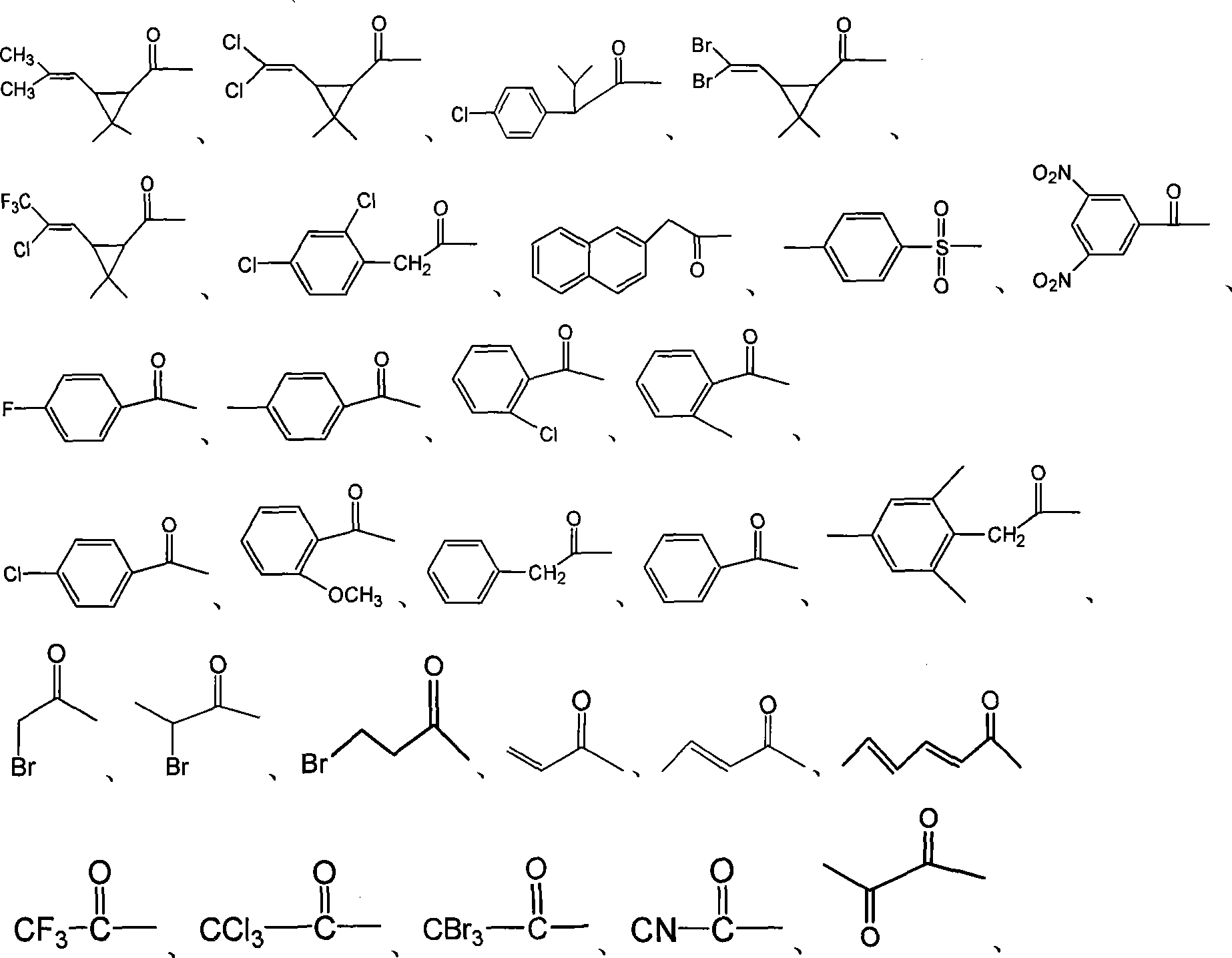
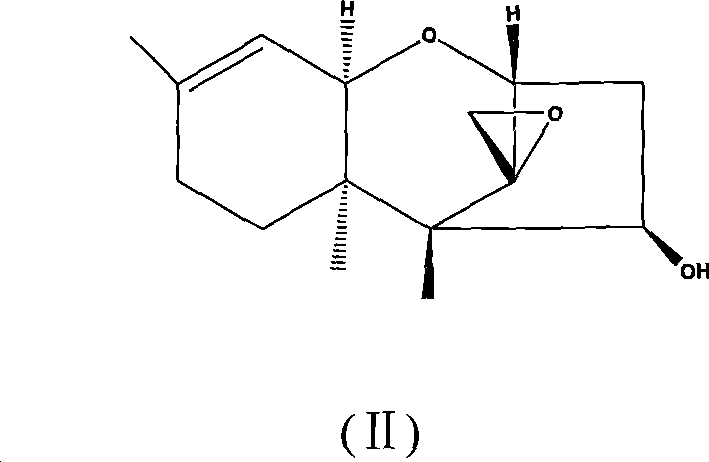
[0055] Add 4.0g (16.0mmol) Trichoderma hydrolyzate (structural formula II), 6.0g (60.0mmol) triethylamine and 80mL dichloromethane in the reaction flask under ice-water bath, then add dropwise 3.6g (19.3mmol) dimethyl Chrysanthemum acid chloride, stirred at room temperature (0-30°C) for 0.5h.
[0056] The reaction solution was washed three times with 1% hydrochloric acid, and then washed with saturated NaHCO 3 The solution was washed twice, and finally washed twice with water, anhydrous Na 2 SO 4 Dry and concentrate to obtain 6.5 g of solid product, which is recrystallized from ethanol and separated by filtration to obtain 5.8 g of product a-1, Y=90.6%. 1 H-NMR (500MHz, CDCl 3 , δppm): 5.596-5.573 (1H, m, -COOCH-), 5.411-5.398 (1H, m, -C=CH-), 4.898-4.877 (1H, m, Me 2 C=CH-), 3.830-3.819 (1H, m, -O-CH-C...
Embodiment 2
[0057] Embodiment 2, a kind of synthetic method of trichomycin derivative c-2, its reaction formula is:
[0058]
[0059] Add 4.0g (16.0mmol) Trichoderma hydrolyzate (structural formula is II), 6.0g (60.0mmol) triethylamine and 80mL dichloromethane in the reaction bottle under ice-water bath, add dropwise 3.28g (19.3mmol) 2- Bromopropionyl chloride, stirred and reacted at room temperature (0-30°C) for 0.5h.
[0060] The reaction solution was washed three times with 1% hydrochloric acid, and then washed with saturated NaHCO 3 The solution was washed twice, and finally washed twice with water, anhydrous Na 2 SO 4 Dry and concentrate to obtain 6.13 g of solid product, which is recrystallized from ethanol and separated by filtration to obtain 5.25 g of product c-2, Y=85.5%. 1 H-NMR (500MHz, CDCl 3 , δppm): 5.589-5.563 (1H, m, -COOCH-), 5.412-5.403 (1H, d, -C=CH-), 4,442-4.393 (1H, m, Br-CH-), 3.853- 3.836 (1H, m, -O-CH-CH 2 -), 3.596-3.585 (1H, d, -C=C-CH-O-), 3.132-3.124...
Embodiment 3
[0061] Embodiment 3, a kind of synthetic method of Trichoderma derivative c-5, its reaction formula is:
[0062]
[0063] Add 4.0g (16.0mmol) Trichoderma hydrolyzate (structural formula is II), 6.0g (60.0mmol) triethylamine and 80mL dichloromethane in the reaction bottle under ice-water bath, then add dropwise 2.0g (19.3mmol) 3, 3-Dimethylbutyryl chloride, stirred and reacted at room temperature (0-30°C) for 6h.
[0064] The reaction solution was washed three times with 1% hydrochloric acid, and then washed with saturated NaHCO 3 The solution was washed twice, and finally washed twice with water, anhydrous Na 2 SO 4 Dry and concentrate to obtain 5.67 g of solid product, which is recrystallized from ethanol and separated by filtration to obtain 4.86 g of product c-5, Y=95.05%. 1 H-NMR (500MHz, CDCl 3 , δppm): 7.036-6.963 (H, m, -CH=CH-CH 3 ), 5.903-5.862 (H, dd, -CH=CH-CH 3 ), 5.630-5.615 (1H, m, -COOCH-), 5.419-5.405 (1H, m, -C=CH-), 3.837-3.827 (1H, d, -O-CH-CH 2 -)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com