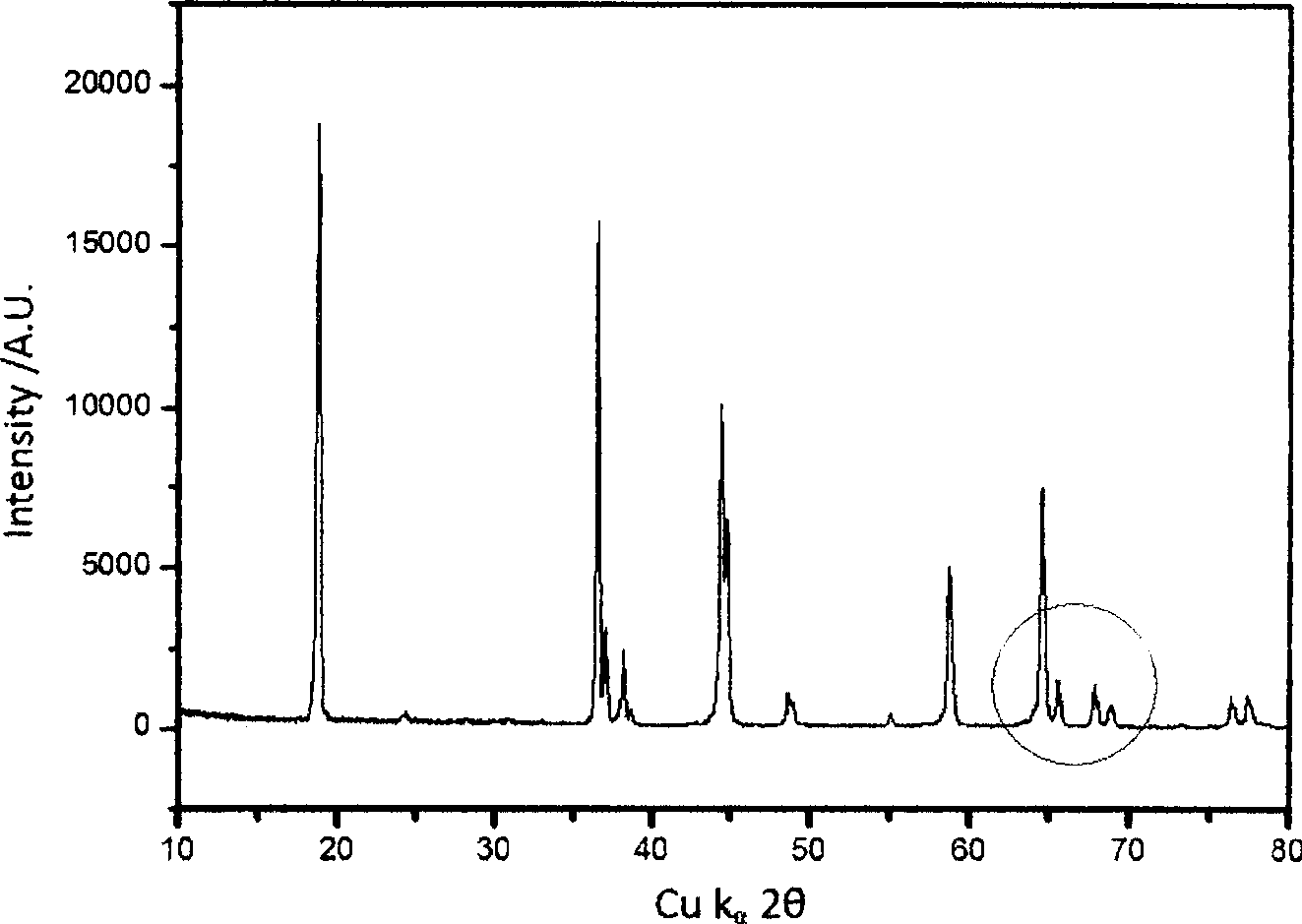
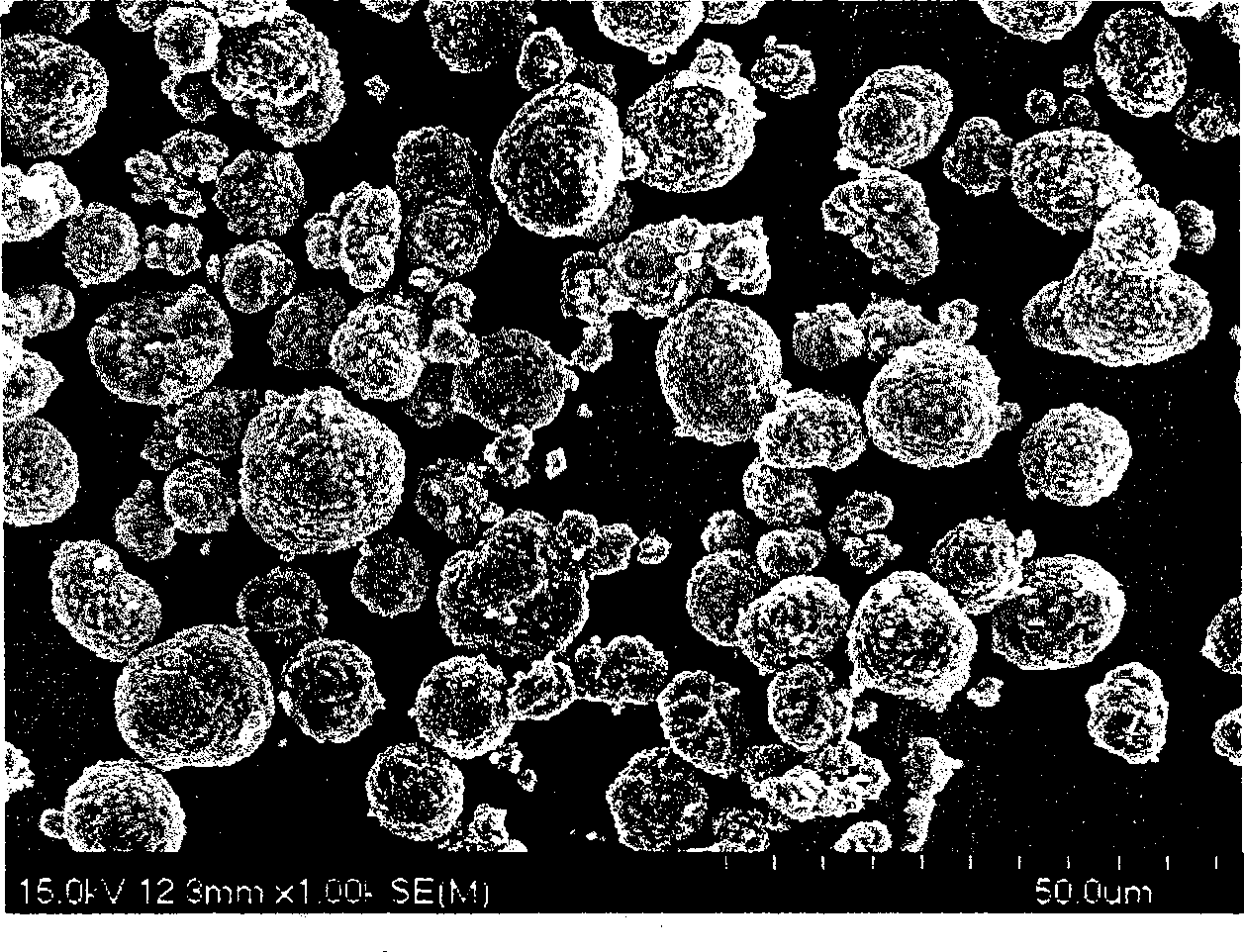
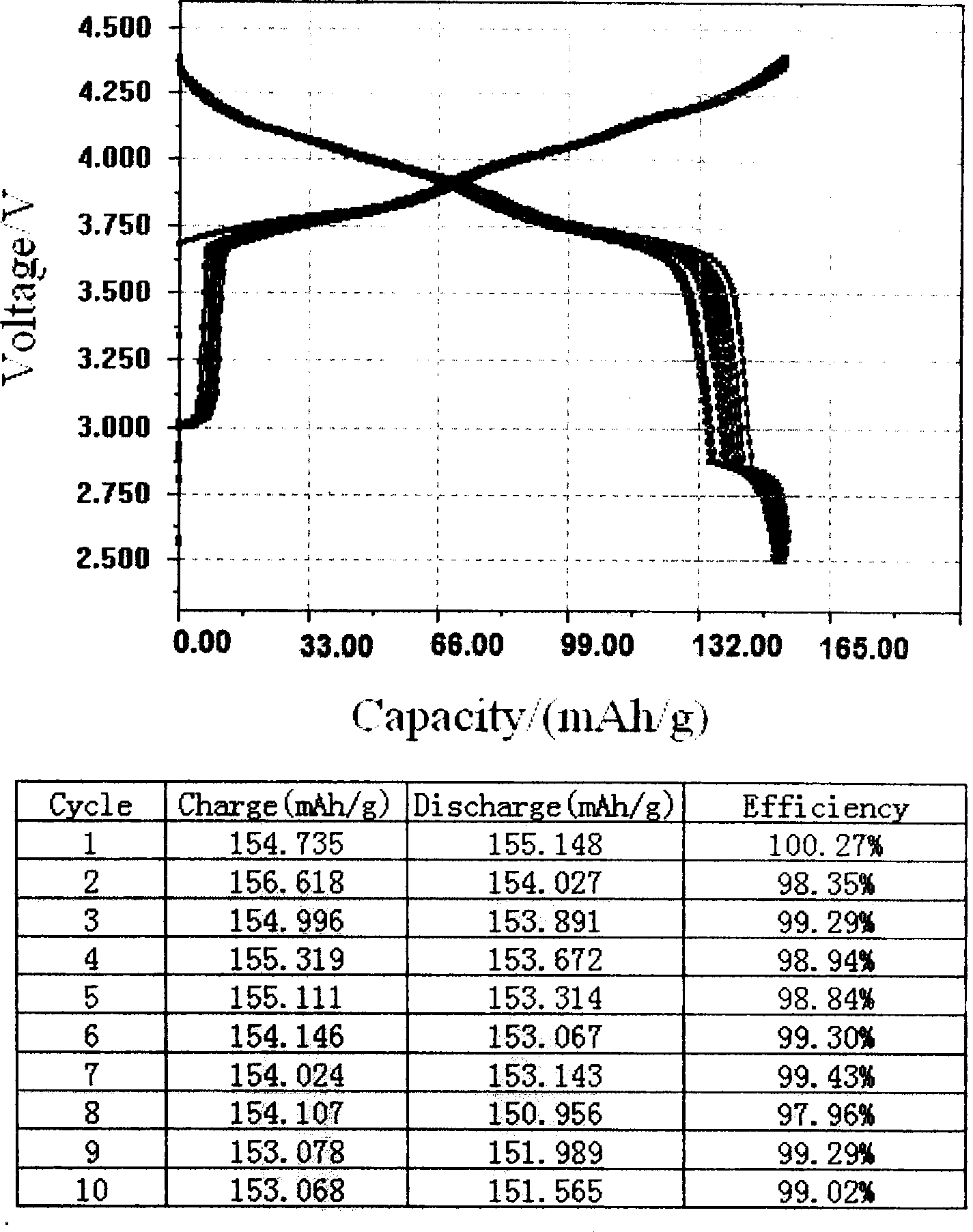
Li-ion battery positive electrode material with layered-spinel symbiotic structure and preparation method
A technology for lithium-ion batteries and positive electrode materials, applied in electrode manufacturing, battery electrodes, structural parts, etc., can solve the problems of lack of cobalt resources and expensive materials, and achieve the solution of lack of cobalt resources, high capacity and cycle performance, and cost reduction Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The product composition is designed with the molar ratio of Co, Ni, Mn=0.2:0.2:0.6.
[0051] Get the raw materials ready: (x is public coefficient in the present embodiment.) MnSO with the MnSO of 0.4xmol earlier 4 .H 2 O prepared into 2mol / L MnSO 4 solution, and then weigh 0.8x mol of NaOH to prepare a 2 mol / L NaOH solution for the first precipitation. Weigh again 0.2x mol of CoSO 4 .7H 2 O, 0.2x mol NiSO 4 .6H 2 O and 0.2x mol of MnSO 4 .H 2 O was prepared into a 2mol / L (Co+Ni+Mn) mixed solution; 1.2x mol of NaOH was weighed to prepare a 2mol / L NaOH solution for the second co-precipitation. The complexing agent uses ammonia water with a concentration of 0.36mol / L.
[0052] making process:
[0053] First, add an appropriate amount of deionized water into the reaction kettle as the bottom liquid of the precipitation reaction, drop in an appropriate amount of ammonia water and NaOH mixed solution to adjust the pH value of the bottom liquid to 10±0.05, and injec...
Embodiment 2
[0060] The product composition is designed with the molar ratio of Co, Ni, and Mn=0.1:0.1:0.8.
[0061] Get the raw materials ready: (x is public coefficient in the present embodiment.) MnSO with the MnSO of 0.7x mol earlier 4 .H 2 O configured as 2mol / L MnSO 4 solution; then weigh 0.14xmol of NaOH to configure a 2mol / L NaOH solution for the first precipitation. Weigh 0.1xmol of CoSO 4 .7H 2 O, 0.1x mol NiSO 4 .6H 2 O and 0.1xmol MnSO 4 .H 2 O was prepared into a 2mol / L (Co+Ni+Mn) mixed solution; 0.6x mol of NaOH was weighed to prepare a 2mol / L NaOH solution for the second step of co-precipitation. The concentration of complexing agent ammonia water is 0.48mol / L.
[0062] making process:
[0063] First, add an appropriate amount of deionized water into the reaction kettle as the bottom liquid of the precipitation reaction, drop in an appropriate amount of ammonia water and NaOH solution to adjust the pH value of the bottom liquid to 10±0.05, and pass N 2 As a protec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com