Tri and tetra-oligo-saccharides suitable as agglutination agents for enteric pathogens
A pathogenic and functional technology, applied in antiviral agents, metabolic diseases, and vector-borne diseases, can solve problems such as heavy economic costs and high medical costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
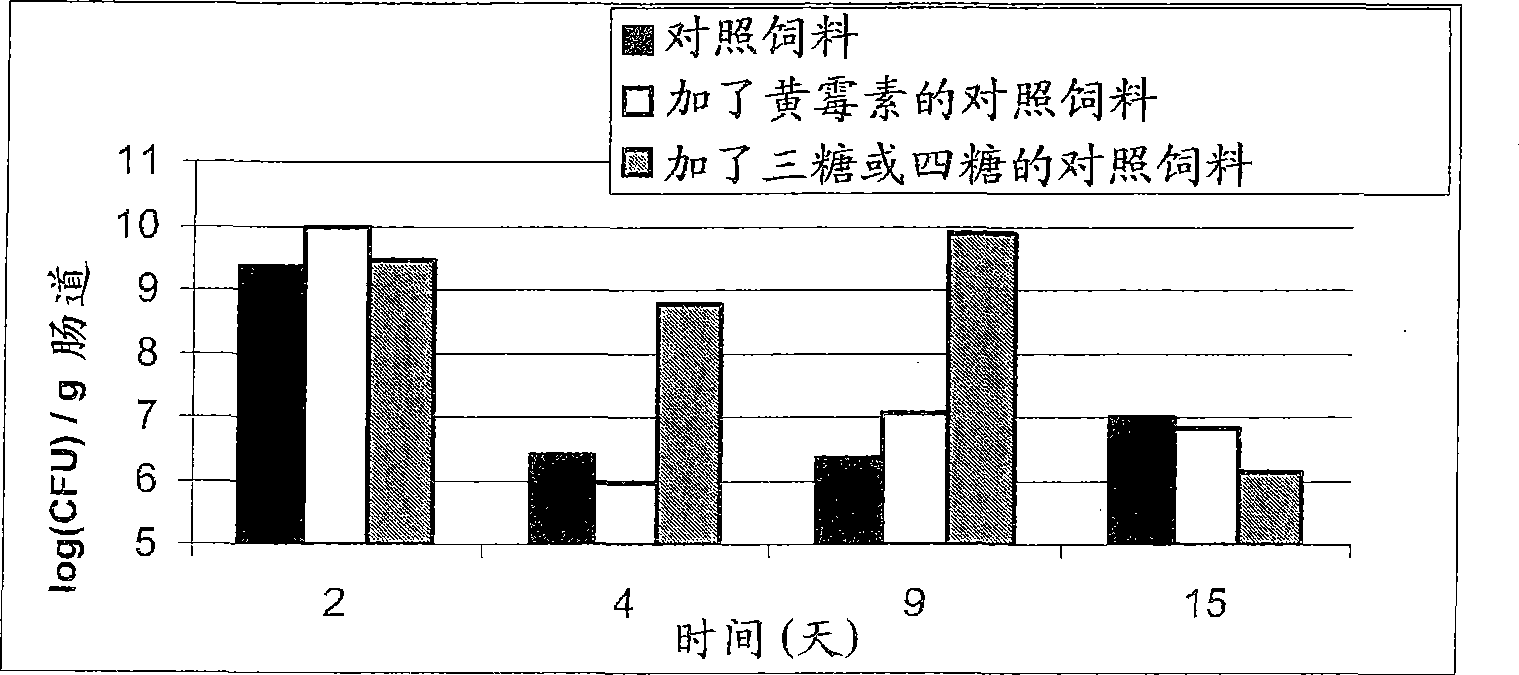
[0048] Example 1: Effects of trimannan oligosaccharides and tetramannan oligosaccharides on microbial ecology in poultry gastrointestinal tract system impact
[0049] 3×40 one-day-old chicks were provided with the following feeds: control feed, control feed supplemented with 3 ppm flavomycin, and control feed supplemented with 0.025% trimannooligosaccharides and / or tetramannoligosaccharides. The control feed was a mashed feed consisting of raw materials suitable as animal nutrition. Water and feed were provided ad libitum. Chicks were infected on day 2 with cecal contents of three-week-old chicks (the period when gastrointestinal problems were most severe). Chicks were dissected at regular intervals and enteropathogen content in the small intestine was determined by plate counting on McFarland agar. The results are summarized in figure 1 middle.
[0050] From figure 1 It is clearly seen that trimannanoligosaccharides and / or tetramannoligosaccharides have a positive ef...
Embodiment 2
[0051] Embodiment 2: Three galacturonic acid oligosaccharides and / or four galacturonic acid oligosaccharides (tri-and / or effect of tetra-galacturonic-oligo-saccharide) on chick performance
[0052] The same experimental conditions as described in Experiment 1 were used. Daily growth and feed conversion were monitored after 13 days in this example. The results are summarized in Table 1.
[0053] Table 1: Effects of Tetraoligosaccharides on Chick Performance
[0054]
[0055] From Table 1 it can be concluded that the use of trigalacturonan oligosaccharides and / or tetragalacturonan oligosaccharides in this particular test gave similar results to those obtained with the traditional growth promoter (flavinomycin). However, the mode of action is not comparable (see Example 1).
Embodiment 3
[0056] Example 3: Trigalacturonic acid oligosaccharides and / or tetragalacturonic acid oligosaccharides on the performance of piglets Impact
[0057] At the beginning of the experiment, 5 piglets were housed in each pen. Each column is equipped with a feed trough (arbitrary assembly) in order to give solid pellet feed. Each column is provided with a drinking nipple. Initially the temperature was 28°C until 10 days after weaning. The temperature was then lowered to 25°C. Administer a commercially available drug-free diet. Drug-free means that the piglets did not receive any therapeutic antibiotics before and during the experiment. The diet is administered in pellet form.
[0058] The diet is as follows:
[0059] -Co: control diet
[0060] -Tr: control diet + 0.125% mannan oligosaccharides
[0061] The experimental design was as follows: 2 treatments (Co and Tr) × 16 parallel determinations × 5 piglets.
[0062] At the start of the experiment, piglets (body weight ap...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com