Method for producing triple superphosphate and co-producing calcium chloride by middle-low grade phosphate rock
A high-grade superphosphate, low-grade technology, applied in chemical instruments and methods, calcium/strontium/barium chloride, phosphorus compounds, etc., can solve the problems of insufficient sulfur resources, tight supply of sulfuric acid, etc., to reduce production costs, sedimentation The effect of fast speed and low impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
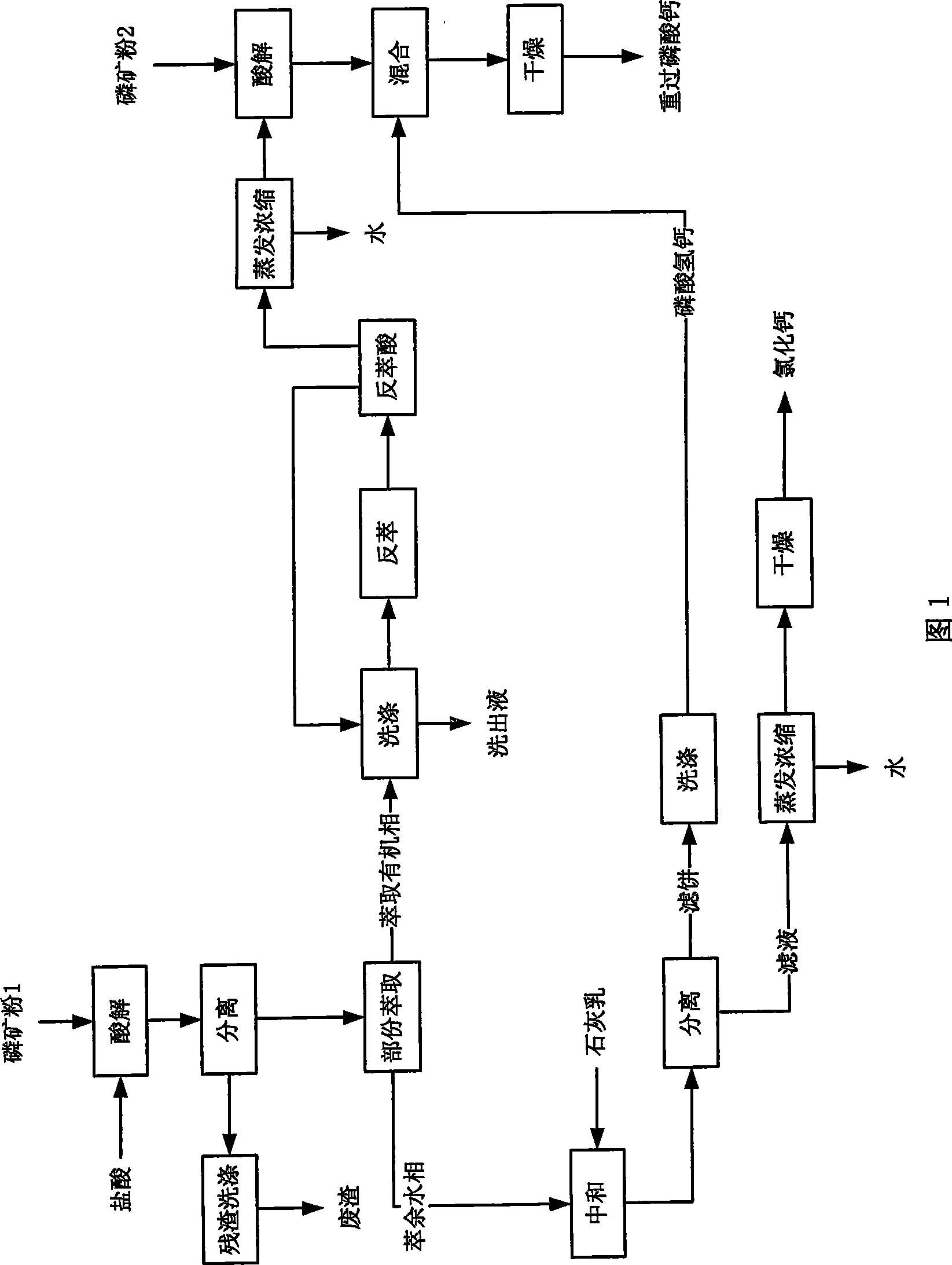
[0040] In the present embodiment, the technological process of the method for producing double superphosphate and co-producing calcium chloride with medium and low-grade phosphate rock is as shown in Figure 2, and the steps are as follows:
[0041] (1) Acid hydrolysis of phosphate rock powder 1
[0042] Phosphate rock powder 1: 1000g, particle size ≤ 1165μm (through 14 mesh sieve), containing 46% CaO, P 2 o 5 24% (mass percentage); Hydrochloric acid: 2500g, mass concentration 24% (by the residue eluate 167g of recovery, the eluate 398g of extracting organic phase and the hydrochloric acid 1935g of mass concentration 31% to be made into, hydrochloric acid consumption is: hydrochloric acid and 100% of the theoretical amount when the CaO in the phosphate rock powder 1 reacts completely);
[0043] During acidolysis, add phosphate rock powder 1 into an acidolysis tank equipped with hydrochloric acid, and react under normal pressure and stirring. The reaction time is 30 minutes. ...
Embodiment 2
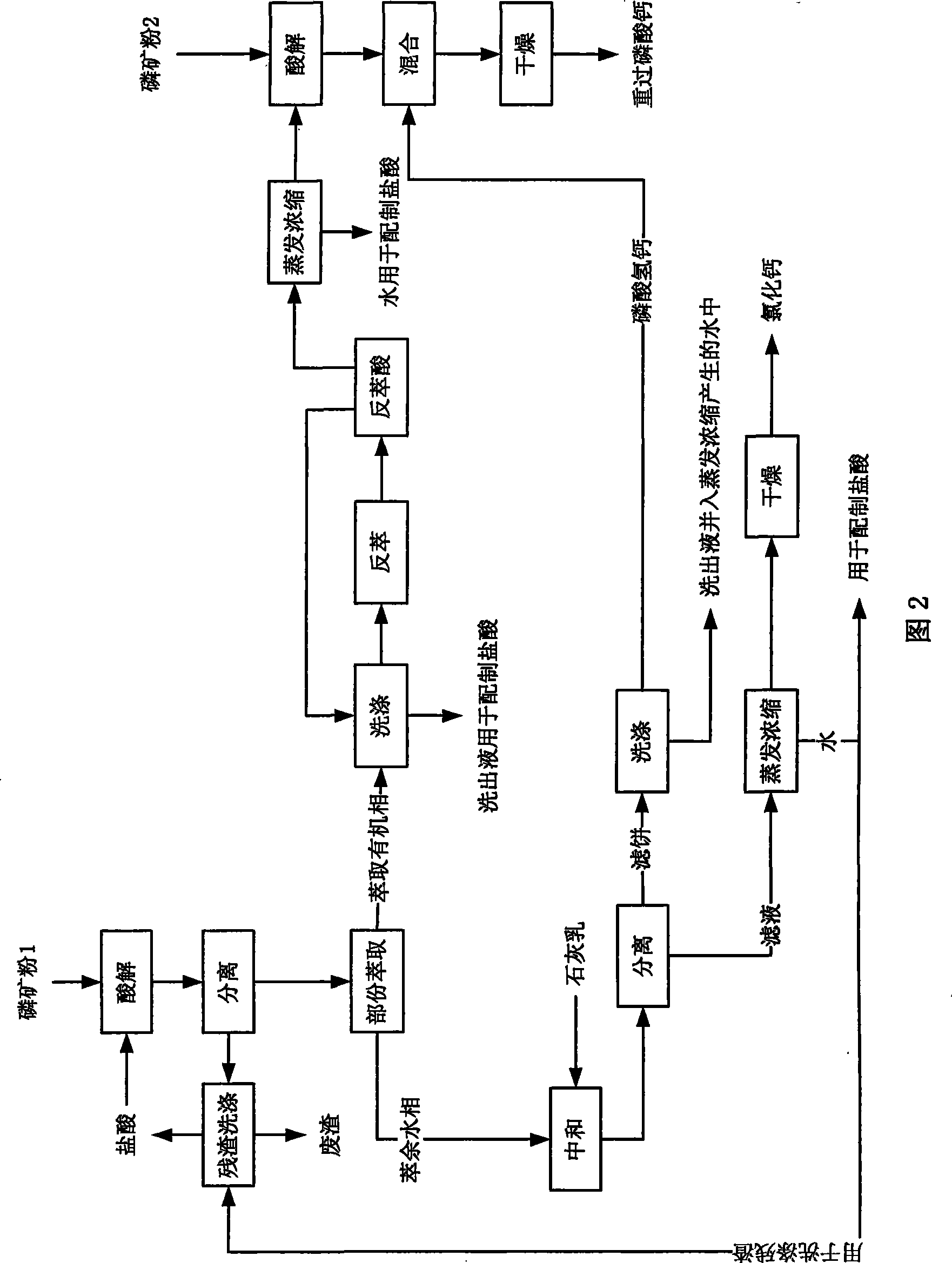
[0059] In the present embodiment, the technological process of the method for producing double superphosphate and co-producing calcium chloride with medium and low-grade phosphate rock is as shown in Figure 2, and the steps are as follows:
[0060] (1) Acid hydrolysis of phosphate rock powder 1
[0061] Phosphate rock powder 1: 1000g, particle size ≤ 1165μm (through 14 mesh sieve), containing 46% CaO, P 2 o 5 24% (mass percentage); Hydrochloric acid: 3314g, mass concentration 19% (by the residue eluent 420g of recovery, the eluate 810g of extraction organic phase, the water 53g that the evaporation concentration process of preparing calcium chloride produces and mass concentration 31 2031g of hydrochloric acid is made into, and the hydrochloric acid consumption is: 105% of theoretical amount when hydrochloric acid and the CaO in the phosphate rock powder 1 react completely);
[0062] During acidolysis, add phosphate rock powder 1 into an acidolysis tank equipped with hydroc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com