Method for chlorides from zinc alkali solution
An alkaline solution and chloride technology, applied in the field of removing chlorides in zinc alkaline solution, can solve problems such as anode plate corrosion, and achieve a remarkable and stable purification effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
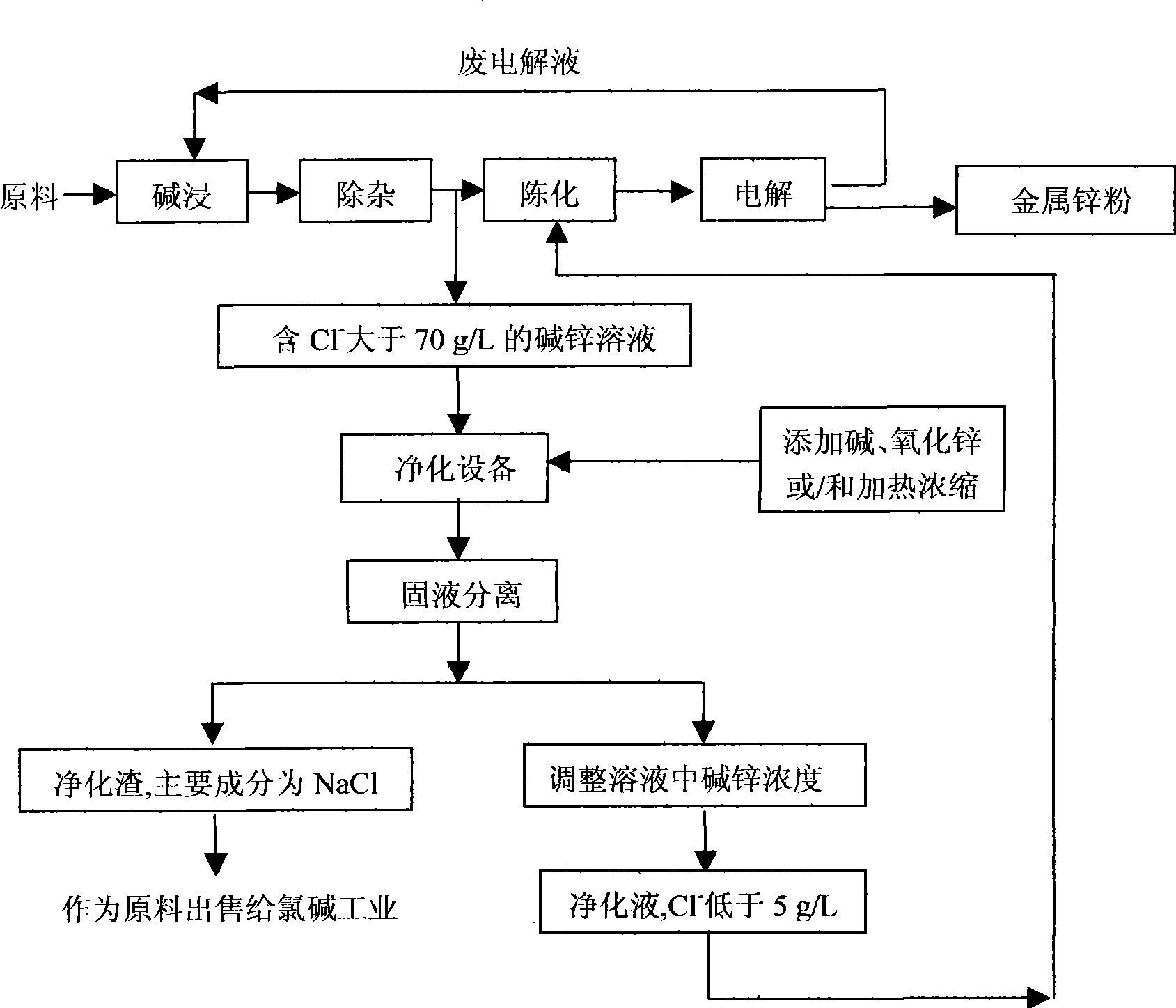
[0015] A certain zinc oxide dust is used as raw material, and the chlorine content is 5.33%. The zinc is extracted by alkali leaching-electrolysis process to produce metal zinc powder, and the lye is recycled. After the number of times of leaching reaches 80 times, the purification solution that has removed metal ions such as lead is analyzed (the composition is shown in Table 1). It can be seen from Table 1 that the chloride ion concentration has reached 75.50g / L and needs to be removed. Take 200 mL of this solution in a 250 mL Erlenmeyer flask, add 90 g of NaOH and 19 g of ZnO (both analytically pure), cover the Erlenmeyer flask with a small funnel (to prevent the solution from volatilizing), heat and stir at 85 ° C for 4 hours, and promote the full dissolution of NaOH and ZnO , then cooled to normal temperature (23°C) and continued to stir for 30 minutes, let it stand for 100 minutes, suction filtered and took the filtrate for analysis. The concentration of NaOH in the solu...
Embodiment 2
[0025] A certain zinc oxide scum is used as raw material, and the chlorine content is 2.65%. The zinc is extracted by alkali leaching-electrolysis process to produce metal zinc powder, and the lye is recycled. After the number of times of leaching reaches 320 times, the purification solution that has removed metal ions such as lead is analyzed (the composition is shown in Table 3). It can be seen from Table 3 that the chloride ion concentration has reached 72.35g / L and needs to be removed. Take 200mL of this solution in a 250mL Erlenmeyer flask, heat and stir at 90°C for 8h to concentrate the solution, cool to room temperature (18°C), continue to stir for 40min, let it stand for 90min, and obtain 75mL of filtrate by suction filtration. Analysis shows that the concentration of NaOH in the solution is 495.65g / L, Zn 94.10g / L, Cl - The concentration dropped from 72.35g / L to 12.85g / L. The resulting filtrate was diluted to 200 mL with distilled water, and the solution was analyze...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com