Liquid crystal tropism agent, liquid crystal tropism film and liquid crystal display element
A liquid crystal alignment agent, general formula technology, used in liquid crystal materials, optics, instruments, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0341] Hereinafter, although an Example demonstrates this invention, this invention is not limited to these Examples. The compounds used in the examples are as follows.
[0342]
[0343] Compound (1): Pyromellitic Dianhydride: PMDA
[0344] Compound (19): 1,2,3,4-cyclobutanetetracarboxylic dianhydride (1,2,3,4-cyclobutanetetracarboxylic dianhydride): CBDA
[0345] Compound (23): 1,2,3,4-butanetetracarboxylic dianhydride: BT
[0346] Compound (37): 1,3,3a,4,5,9b-hexahydro-5(tetrahydro-2,5-dioxo-3-furyl)naphtho[1,2-c]furan-1 , 3-diketone (1,3,3a,4,5,9b-hexahydro-5(tetrahydro-2,5-dioxo-3-furanyl)naphoto[1,2-c]furan-1,3-dione) : TDA
[0347] Compound (49): 2,3,5-tricarboxycyclopentyl acetic dianhydride (2,3,5-tricarboxycyclopentyl acetic dianhydride): TCMP
[0348]
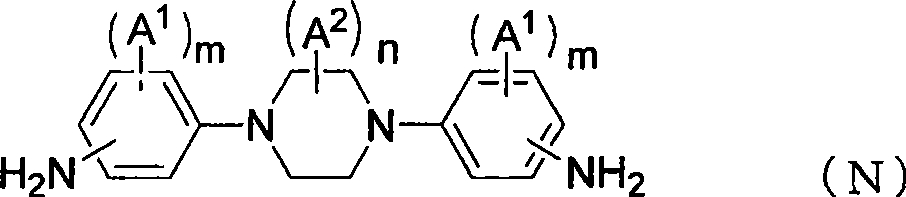
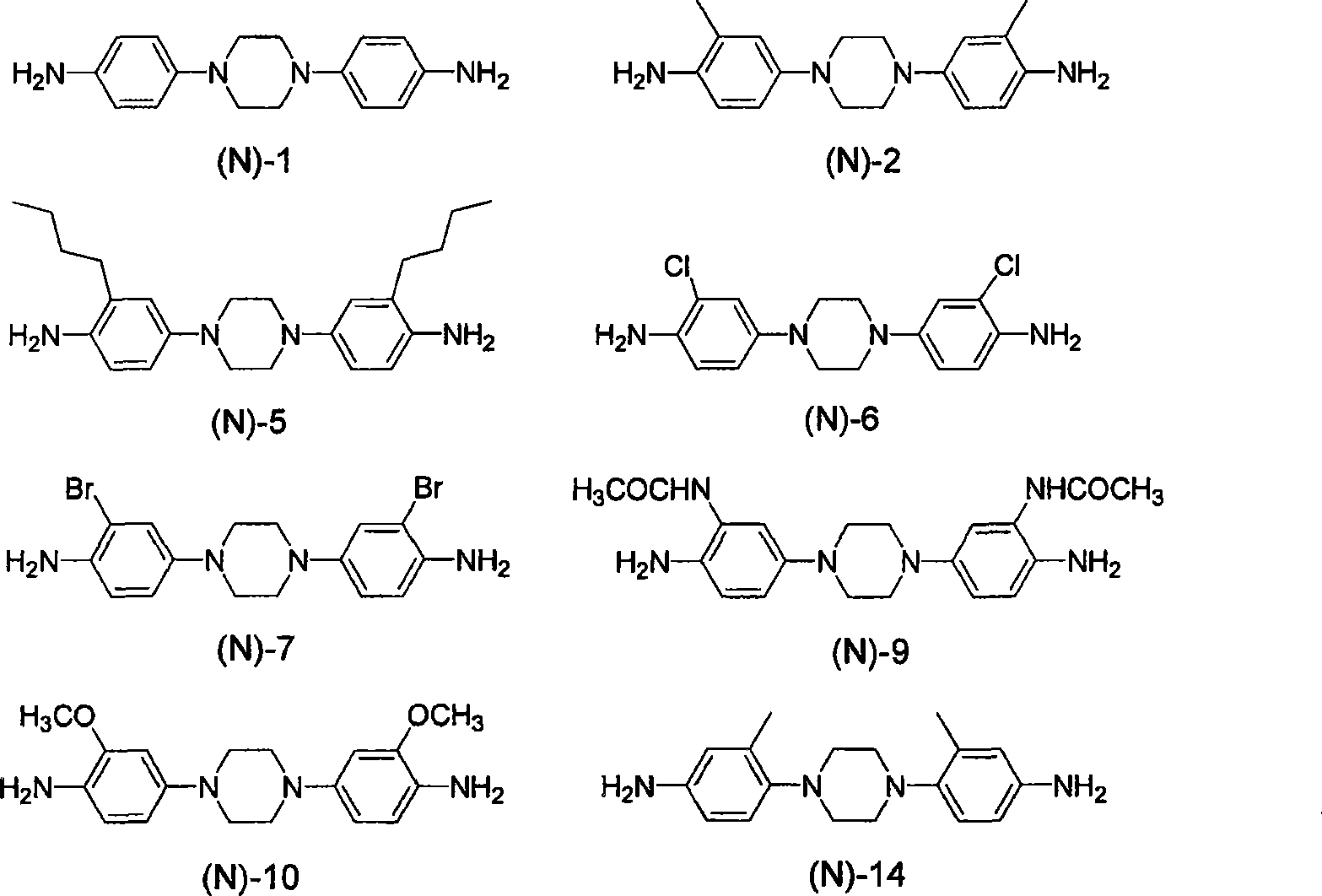
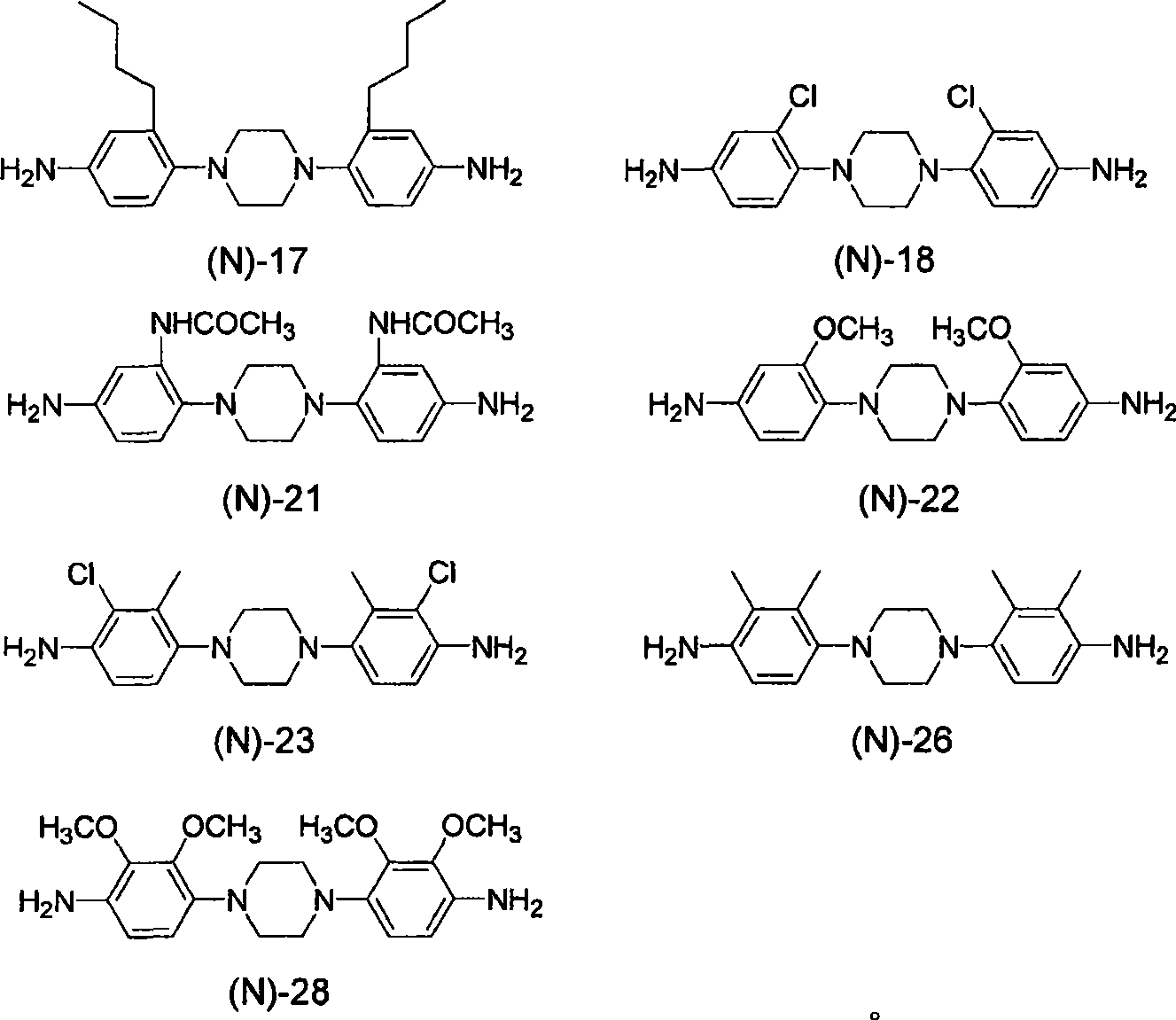
[0349] Compound: 1,4-bis(4-aminophenyl)-1,4-diazacyclohexane (1,4-bis(4-aminophenyl)-1,4-diazacyclohexane): DAC
[0350] Compound: 4,4'-(piperazine-1,4-diyl)bis(2-methylaniline): 3MPDA
[0351] Compound: 1,...
Synthetic example 1
[0374] Into a 100 mL four-necked flask equipped with a thermometer, a stirrer, a raw material input port, and a nitrogen gas introduction port, 3.651 g of compound DAC and 54.0 g of dehydrated NMP were added, and stirred and dissolved under a dry nitrogen stream. Next, 1.237 g of compound (1), 1.112 g of compound (19) and 15.0 g of dehydrated GBL were added and reacted at room temperature for 30 hours. When the reaction temperature rises during the reaction, the reaction is carried out by suppressing the reaction temperature to about 70°C or lower. Then, 25.0 g of BC was added to the obtained solution to obtain a polyamic acid solution having a concentration of 6% by weight. Let this polyamic acid be PA1. The weight average molecular weight of PA1 was 34,800.
[0375] The weight-average molecular weight of polyamic acid is obtained in the following manner: the obtained polyamic acid is diluted with a phosphoric acid-DMF mixed solution (phosphoric acid / DMF=0.6 / 100: weight rat...
Synthetic example 2
[0376] [Synthesis Example 2 to Synthesis Example 31]
[0377] Except changing tetracarboxylic dianhydride and diamine as shown in Table 1 and Table 2, polyamic-acid solution (PA2) - polyamic-acid solution (PA31) were prepared according to the synthesis example 1. Including Synthesis Example 1, the results are summarized in Table 1 and Table 2. In Synthesis Example 24 to Synthesis Example 28, polyamic acid could not be synthesized because a precipitate was deposited during the reaction.
[0378] [Table 1]
[0379] Synthesis example
[0380] [Table 2]
[0381] Synthesis example
[0382]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com