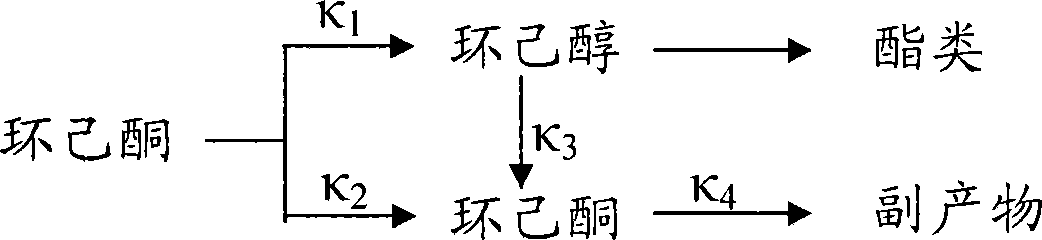
Method for preparing cyclohexanol and cyclohexanone by catalytic oxidation of cyclohexane
A technology for catalytic oxidation and cyclohexane, applied in the field of catalytic oxidation of cyclohexane to prepare cyclohexanol and cyclohexanone, can solve the problem that high cyclohexane conversion rate and high alcohol ketone selectivity cannot be achieved at the same time, and industrial production cannot be achieved. requirements, low conversion of cyclohexane, etc., to achieve the effect of maintaining low temperature, high activity, high stereoselectivity, and controlling catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] In a 2000 ml glass flask, add 1000 ml of acetonitrile, 0.3 g of heteropolyacid catalyst H 2 [V 4 (H 2 O) 9 (PW 9 o 34 ) 2 ], add 14 milliliters of 30% (mass percentage concentration) aqueous hydrogen peroxide solution, stir and reflux at room temperature for half an hour, then add 20 milliliters of hexanaphthene, increase the temperature value to 80 ° C, slowly add the remaining 30% (mass percent concentration) hydrogen peroxide aqueous solution (55 milliliters), constant temperature reaction 9-12 hour, reduce temperature to room temperature, GC analysis mixture, cyclohexane transformation rate is 98%, and the total selectivity of cyclohexanol and cyclohexanone is 97%. The ratio of cyclohexanol to cyclohexanone is 1:2.
Embodiment 2
[0049] In a 2000 ml glass flask, add 1000 ml of water and 0.3 g of heteropolyacid catalyst H 2 [V 4 (H 2 O) 9 (PW 9 o 34 ) 2 ], add 14 milliliters of 30% (mass percentage concentration) aqueous hydrogen peroxide solution, stir and reflux at room temperature for half an hour, then add 20 milliliters of hexanaphthene, increase the temperature value to 80 ° C, slowly add the remaining 30% (mass percentage concentration) hydrogen peroxide aqueous solution (55ml), constant temperature reaction 9-12 hour, reduce temperature to room temperature, GC analysis mixture, cyclohexane conversion efficiency is 91%, and the total selectivity of cyclohexanol and cyclohexanone is 98 %. The ratio of cyclohexanol to cyclohexanone is 1:1.
Embodiment 3
[0051] In a 2000 ml glass flask, add 1000 ml of dichloroethane, 0.3 g of heteropolyacid catalyst H 2 [V4 (H 2 O) 9 (PW 9 o 34 ) 2 ], add 14ml of 30% (mass percent concentration) hydrogen peroxide aqueous solution, stir and reflux at room temperature for half an hour, then add 20 milliliters of hexanaphthene, increase the temperature value to 80 ° C, slowly add dropwise the remaining (55ml) 30% (mass percentage concentration) hydrogen peroxide aqueous solution, constant temperature reaction 9-12 hours, reduce temperature to room temperature, GC analysis mixture, cyclohexane conversion rate is 90%, the total selectivity of cyclohexanol and cyclohexanone is 97% . The ratio of cyclohexanol to cyclohexanone is 1.2:1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com