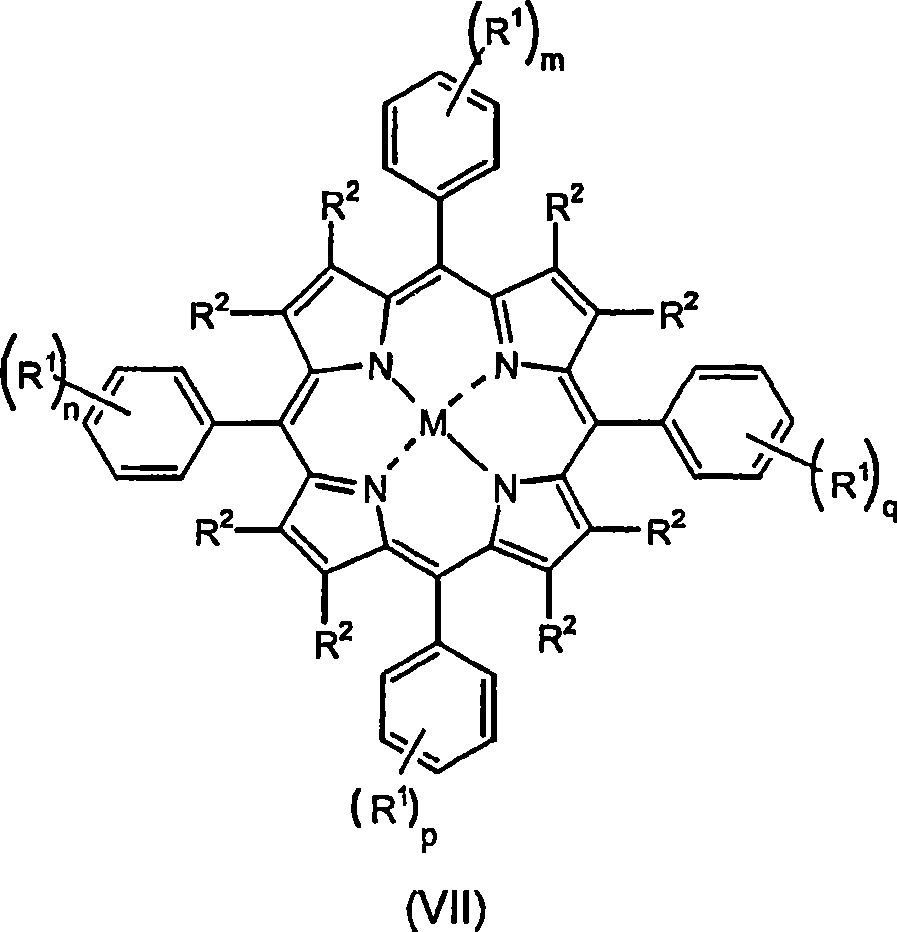
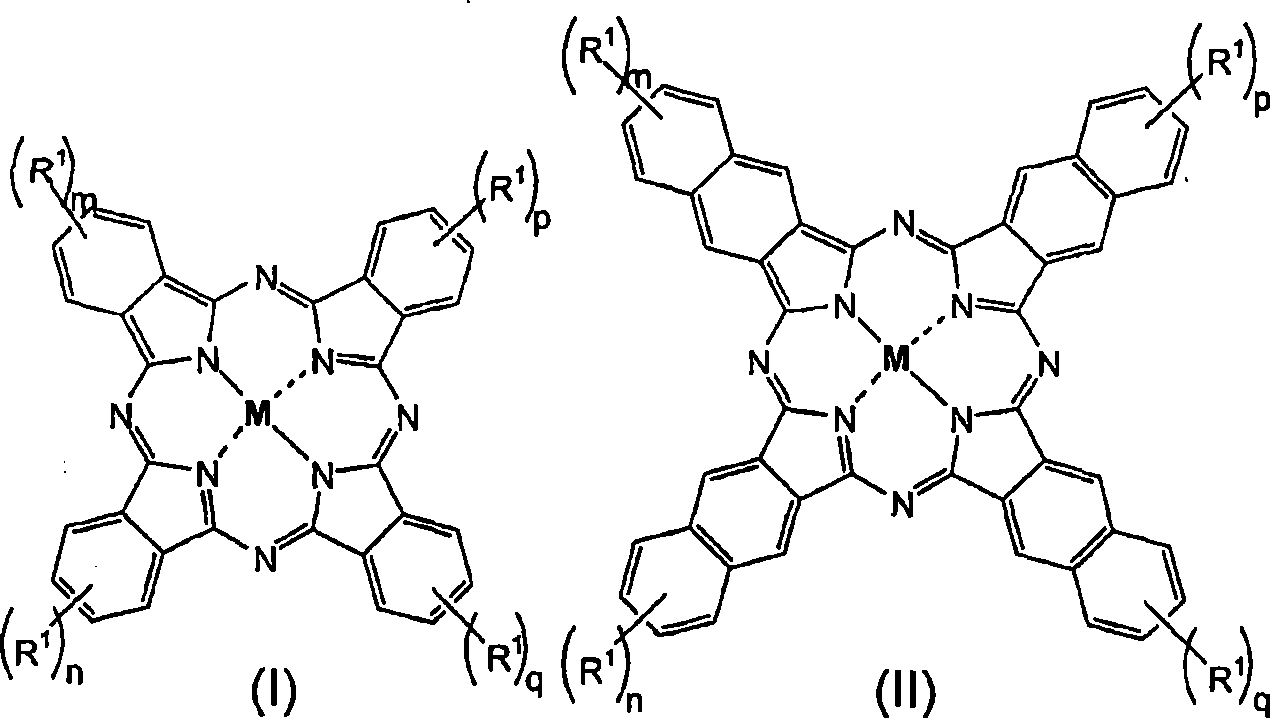
Fluorinated dyes or colorants and their uses
A colorant, fluorinated solvent technology, applied in organic dyes, azo dyes, organic chemistry, etc., can solve the problems of limited solubility and high viscosity of electrophoretic dispersions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
[0185] Preparation of Substituted Copper Phthalocyanine (C3)
[0186]
[0187] m, n, p and q are independently 0-4, and m+n+p+q≤4
[0188] C3
[0189] Copper phthalocyanine (1.4gm, 2.4mmole, Aldrich) and Krytox A mixture of iodides (16.32 gm, 9.6 mmole, DuPont) was charged to a 72 mL glass-lined pressure reactor (Parr Instruments). The reactor was vacuum sealed at 1 Torr and heated at 350°C for 38 hours. Use 250 mL of PFS-2 in a Soxhlet extractor TM The resulting crude product was extracted (extracted) for 1 day. The dark blue solution thus obtained was washed 3 times with 250 mL of acetone and evaporated to a slurry by rotary evaporation (60 °C) under vacuum (~5 Torr) and then under high vacuum (~1 Torr) overnight. The syrupy compound was further purified by refluxing with diethyl ether (250 mL) for 1 day and the resulting mixture was separated. The dark blue syrup was evaporated again by rotary evaporation (60 °C) under vacuum (~5 Torr) and then under high vacuum...
Embodiment 12
[0191] Preparation of Substituted Copper Tetra-tert-butyl Phthalocyanine (CB2)
[0192]
[0193] m, n, p and q are independently 0-3, and m+n+p+q≤2 or 3
[0194] CB2
[0195] 1. Synthesis of copper(II) bromide 2,9,16,23-tetra-tert-butyl-29H,31H-phthalocyanine
[0196] At room temperature, in trifluoroacetic acid (50mL, Aldrich) and H 2 SO 4 (15 mL, Fisher Scientific) was stirred with copper(II) 2,9,16,23-tetra-tert-butyl-29H,31H-phthalocyanine (1.00 gm, 1.24 mmol, Aldrich) and N-bromosuccinimide (1.00 gm, 5.61 mmol, Aldrich) for 24 hours. The resulting dark blue solution was poured into 200 mL of ice water. The resulting solid was washed with water, removed by filtration, and dried (60°C, 60 Torr) overnight. A dark blue solid (1.22 gm, 86%) was obtained.
[0197] 2. Synthesis of Substituted Copper(II) Tetra-tert-Butyl Phthalocyanine (CB2)
[0198] Add 25 mL (11.25 mmole) of a 0.45-0.50 M solution of 9-boronbicyclo[3.3.1]nonane (9-BBN) in THF into 4 gm of Kr...
Embodiment 13
[0200] Preparation of Substituted Copper Tetrakis(4-cumylphenoxy)phthalocyanine (CC1)
[0201]
[0202] m, n, p and q are independently 0-4, and m+n+p+q≤2 or 3
[0203] CC1
[0204] 1. Synthesis of Copper(II) Bromide Tetrakis(4-cumylphenoxy)phthalocyanine
[0205] At room temperature, in trifluoroacetic acid (50mL, Aldrich) and H 2 SO 4 (15mL, Fisher Scientific) was stirred in a solution of copper(II) tetrakis(4-cumylphenoxy)phthalocyanine (1.00gm, 0.71mmol, Aldrich) and N-bromosuccinimide (0.60gm, 3.37mmol, Aldrich) mixture for 24 hours. The resulting dark blue solution was poured into 200 mL of ice water. The resulting solid was washed with water, removed by filtration, and dried (60°C, 60 Torr) overnight. A dark blue solid (1.10 gm, 89%) was obtained.
[0206] 2. Synthesis of substituted copper tetrakis(4-cumylphenoxy)phthalocyanines (CC1) to make
[0207] At 0°C, 25mL (11.25mmol) of 9-borabicyclo[3.3.1]nonane (9-BBN) 0.45-0.50M solution in tetrahydrofur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com