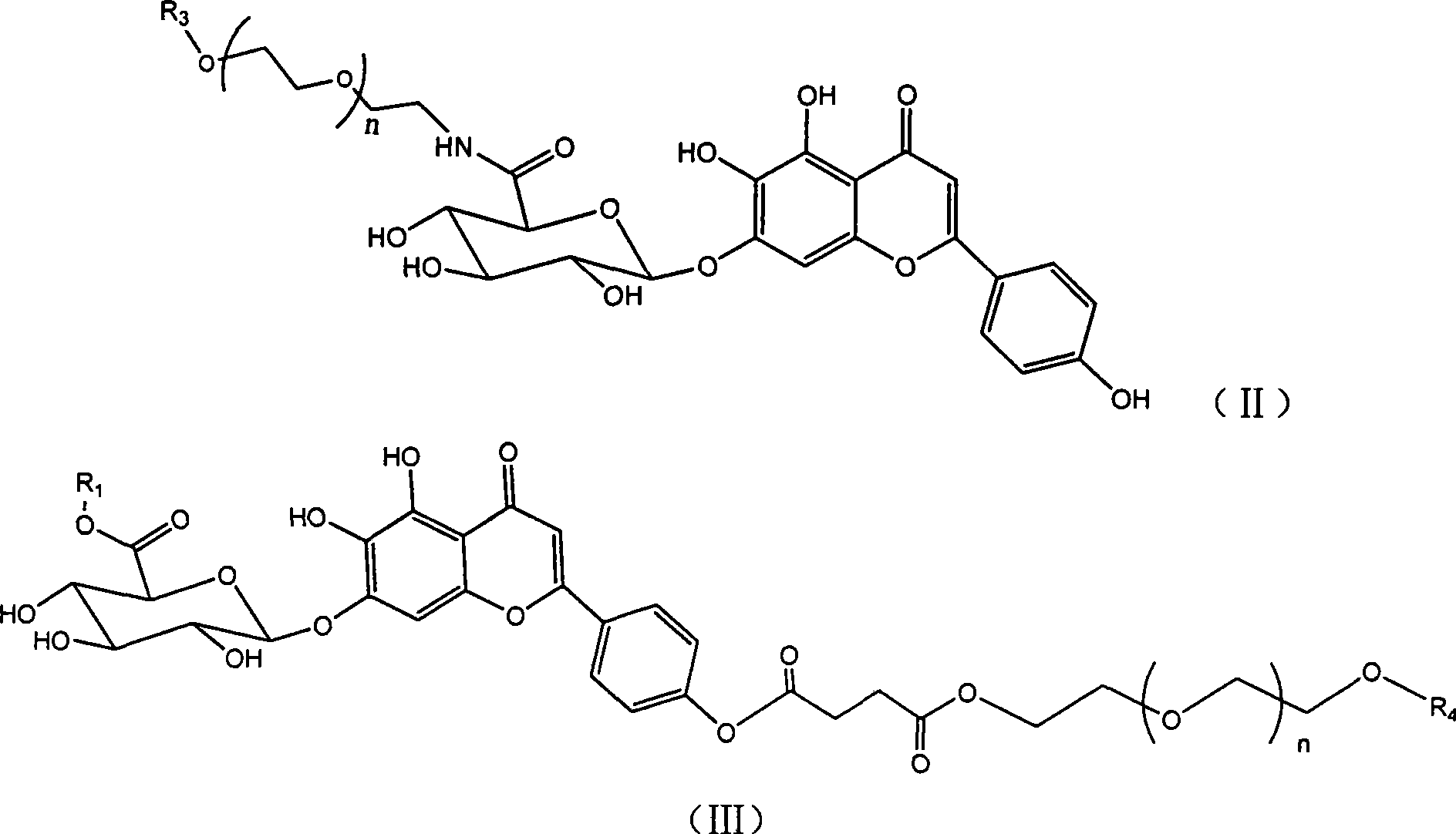
Polyethyleneglycol modified scutellarin compound and preparation thereof
A technology of scutellarin and polyethylene glycol, which is applied in the field of PEG-modified prodrugs and its preparation, can solve the problems of research, low solubility, no pharmacokinetics and pharmacodynamics, etc., to improve water solubility, The effect of enhancing biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
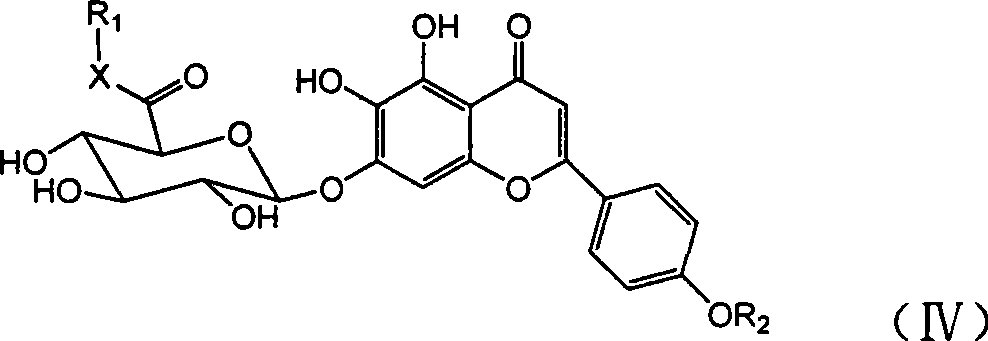
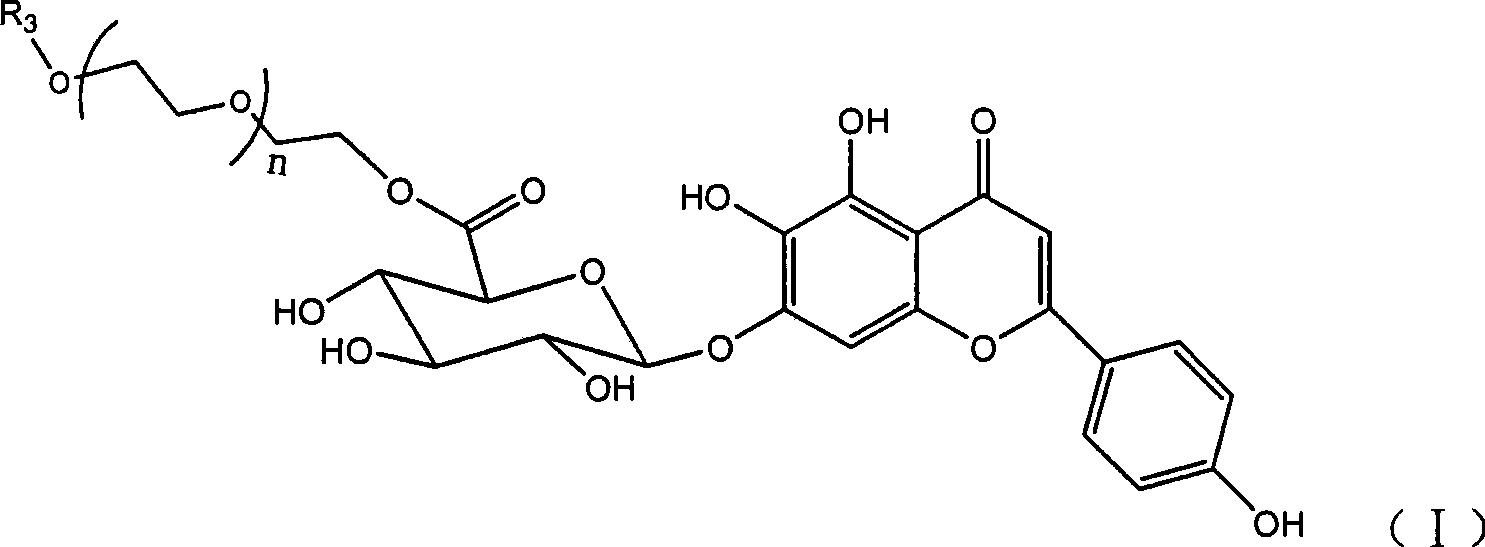
[0110] Example 1: Synthesis of scutellarin-polyethylene glycol (400) monomethyl ether-6 "-ester (compound I-400)
[0111]
[0112] Add 9.24g of scutellarin, 12g of mPEG(400), 4.05g of HOBt, and 6.18g of DCC (molar ratio 1:1.5:1.5:1.5) into 80mL of DMF to dissolve, protect the reaction system with nitrogen gas, and heat to 40°C Reacted for 6 hours, and TLC detected the reaction end point (CH 2 Cl 2 :CH 3 OH:HCOOH=10:1:0.2), after the reaction is completed, add water to terminate the reaction, filter out part of the solid, concentrate the filtrate under reduced pressure to remove DMF and water, and the thick product of the viscous material is subjected to CH 2 Cl 2 :CH 3 The eluent of OH:HCOOH=10:1:0.1 was separated and purified through a silica gel column for three times to obtain 7.65 g of a light yellow powdery solid with a yield of 45.3%.
[0113] 1H NMR (DMSO-d6, 300MHz): δH 6.81 (1H, S, C 3 -H), 7.00 (1H, S, C 8 -H), 7.93 (2H, d, J=8.9Hz, C 2’,6’ -H), 6.96 (2H, ...
Embodiment 2
[0115] Example 2: Synthesis of scutellarin-polyethylene glycol (600) monomethyl ether-6 "-ester (compound I-600)
[0116] Add 13.86g of scutellarin, 27g of mPEG(600), 6.07g of HOBt, and 9.27g of DCC (molar ratio 1:1.5:1.5:1.5) into 120mL of DMF to dissolve, protect the reaction system with nitrogen gas, and heat to 45°C Reacted for 8 hours, and TLC detected the reaction end point (CH 2 Cl 2 :CH 3 OH:HCOOH=10:1:0.2), after the reaction is completed, add water to terminate the reaction, filter out part of the solid, concentrate the filtrate under reduced pressure to remove DMF and water, and pass the viscous material crude product through CH 2 Cl 2 :CH 3 The eluent of OH:HCOOH=10:1:0.1 was separated and purified through a silica gel column for three times to obtain 12.62 g of a light yellow powdery solid with a yield of 40.3%.
[0117] 1 H NMR (DMSO-d6, 300MHz): δH 6.81 (1H, S, C3-H), 7.00 (1H, S, C 8 -H), 7.93 (2H, d, J=8.9Hz, C 2’,6’ -H), 6.96 (2H, d, J=8.9Hz, C 3’,5’...
Embodiment 3
[0119] Example 3: Synthesis of scutellarin-polyethylene glycol (1000) monomethyl ether-6 "-ester (compound I-1000)
[0120] Add 9.24g of scutellarin, 30g of mPEG(1000), 4.05g of HOBt, and 6.18g of DCC (molar ratio 1:1.5:1.5:1.5) into 150mL of DMF to dissolve, protect the reaction system with nitrogen gas, and heat to 50°C Reacted for 10 hours, and TLC detected the reaction end point (CH 2 Cl 2 :CH 3 OH:HCOOH=10:1:0.2), after the reaction is completed, add water to terminate the reaction, filter out part of the solid, concentrate the filtrate under reduced pressure to remove DMF and water, and pass the viscous crude product through CH 2 Cl 2 :CH 3 The eluent of OH:HCOOH=10:1:0.1 was separated and purified through a silica gel column for three times to obtain 10.54 g of a light yellow powdery solid with a yield of 36.5%.
[0121] 1 H NMR (DMSO-d6, 300MHz): δH 6.82 (1H, S, C3-H), 7.03 (1H, S, C8-H), 7.94 (2H, d, J=8.9Hz, C2', 6'- H), 6.95 (2H, d, J=8.9Hz, C3', 5'-H), 5.27 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com