Acarbose chewing tablets
A technology of acarbose and tablet cores, applied in the field of medicine, can solve problems such as hardening, affecting taste and efficacy, and easy moisture absorption of acarbose
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
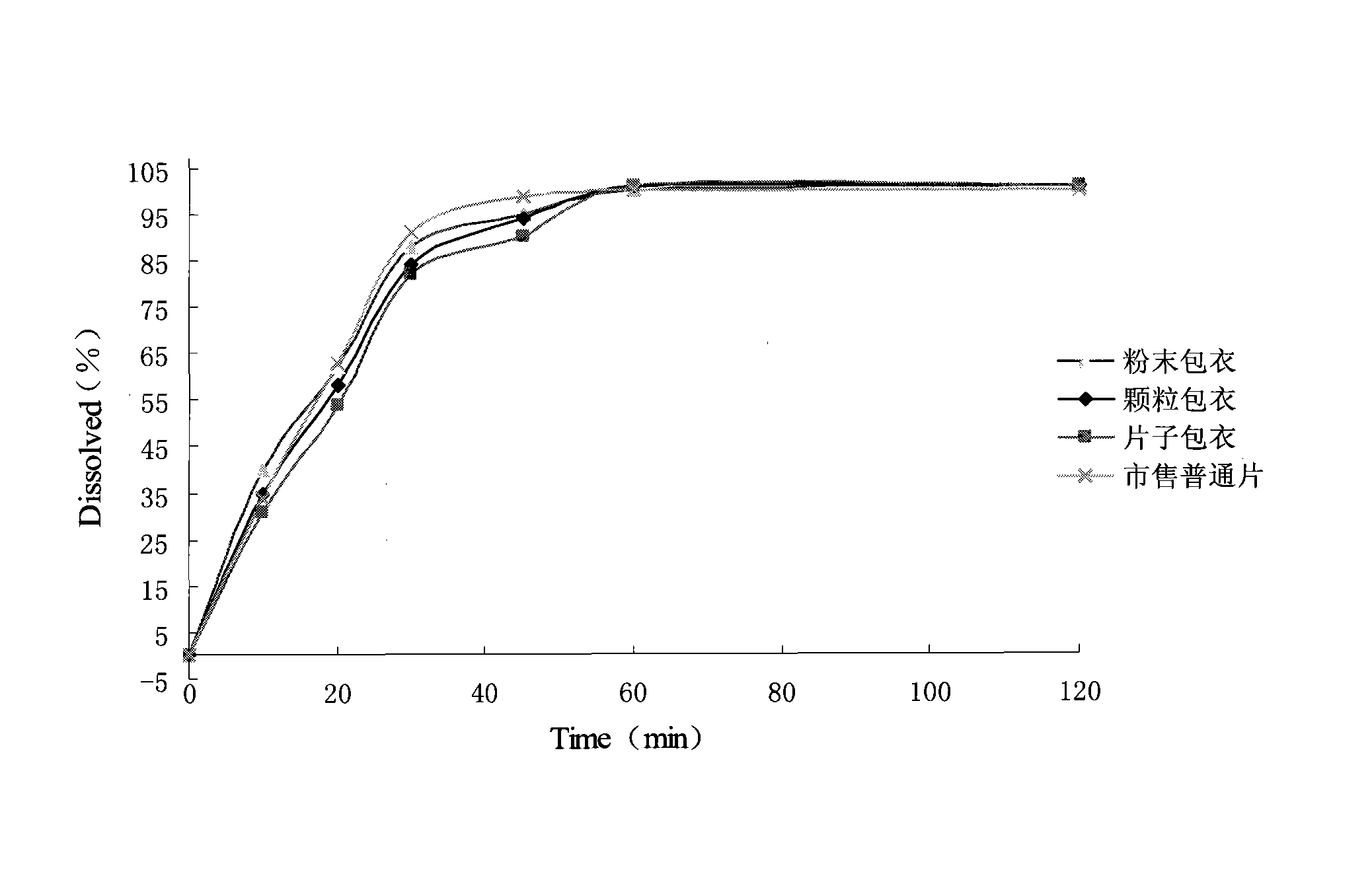
Image
Examples
Embodiment 1
[0029] Embodiment 1 powder coating
[0030] Prescription: (1000 tablets / grain)
[0031] Acarbose 100g
[0032] Opadry AMB 20g
[0033] Xylitol 200g
[0034] Process: add Opadry AMB to 95% ethanol and stir to prepare a coating solution with a concentration of 5-15%, which is used as a coating solution for later use.
[0035] Put the acarbose into the fluidized bed and adopt the top spraying process. Preheat the fluidized bed temperature to 40°C. Spray the above coating solution at a rate of 3.5-5g per minute, the air flow rate is 8-15Hz, and the spray compressed air pressure is 1bar. The coating process depends on controlling the inlet air temperature to maintain the product temperature At 30°C-40°C. After the coating solution is sprayed, the product temperature is maintained at 40°C and dried for 30-60 minutes, and the weight gain is controlled at 5-40%.
[0036] After coating, the powder is evenly mixed with xylitol, and compressed into tablets, chewable tablets or fill...
Embodiment 2
[0037] Embodiment 2 particle coating
[0038] Prescription: (1000 tablets / grain)
[0039] Acarbose 100g
[0040] Acrylic resin E100 10g
[0041] Hydrogenated Vegetable Oil 20g
[0042] Xylitol 200g
[0043] Glyceryl Behenate 3g
[0044] Process: add acrylic resin E100 into 95% ethanol and stir until dissolved, add hydrogenated vegetable oil into hot isopropanol and stir until dissolved, mix the two to form a coating solution with a concentration of 5-15%, and use it as a coating solution for later use.
[0045] Acarbose and xylitol are mixed and added with 95% ethanol solution to make soft material, granulated through 30 mesh, dried, and granulated through 20 mesh. The granules are placed in a fluidized bed, using a top spray process. Preheat fluidized bed temperature to 40 DEG C. Spray with the speed of per minute 3.5-5g with the coating liquid in embodiment 1, air velocity is 8-15Hz, spray compressed air pressure is 1bar. Coating process is by controlling inlet air tem...
Embodiment 3
[0047] Embodiment 3 tablet coating
[0048] Tablet core prescription: (1000 tablets)
[0049] Acarbose 100g
[0050] Microcrystalline Cellulose 193g
[0051] Aspartame 3g
[0053] Silica 2g
[0054] Coating prescription: (1000 tablets)
[0055] Opadry A MB 10g
[0056] 95% ethanol 100g
[0057] Process: add Opadry AMB to 95% ethanol and stir to prepare a coating solution with a concentration of 5-15%, which is used as a coating solution for later use.
[0058] Acarbose, microcrystalline cellulose and aspartame are mixed and added with 95% ethanol solution to make soft material, granulated through 30 mesh, dried, and granulated through 20 mesh. Magnesium stearate and silicon dioxide are added, mixed and compressed into tablets.
[0059] The tablet core is put into a coating pot, coated with the above coating solution, aged in the pot at 50°C for 10-40 minutes after coating, and the weight gain of the coating is controlled at 1-5%. Detailed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com