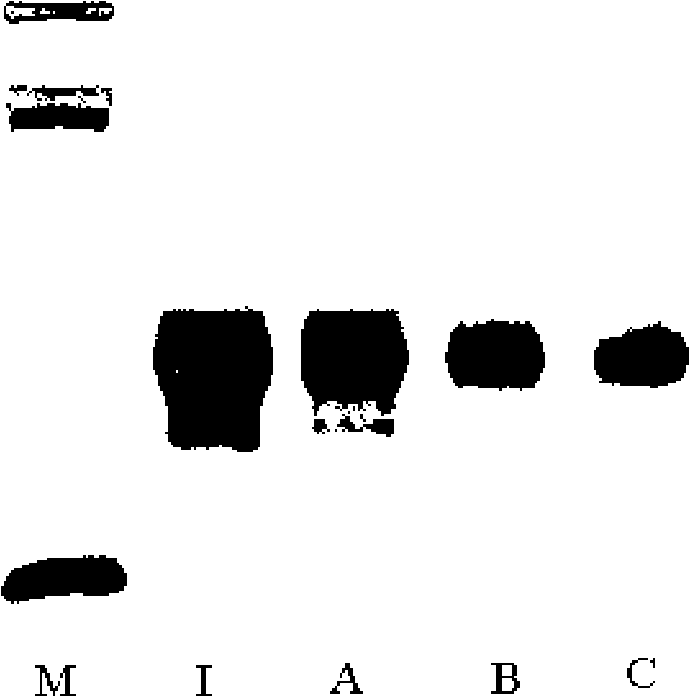PEG-IFN omega conjugate and preparation technique thereof
A technology of recombinant human interferon and polyethylene glycol, applied in the direction of drug combination, digestive system, medical preparations of non-active ingredients, etc., can solve the problems of short half-life, poor stability, etc., and achieve improved uniformity, easy acceptance, The effect of easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
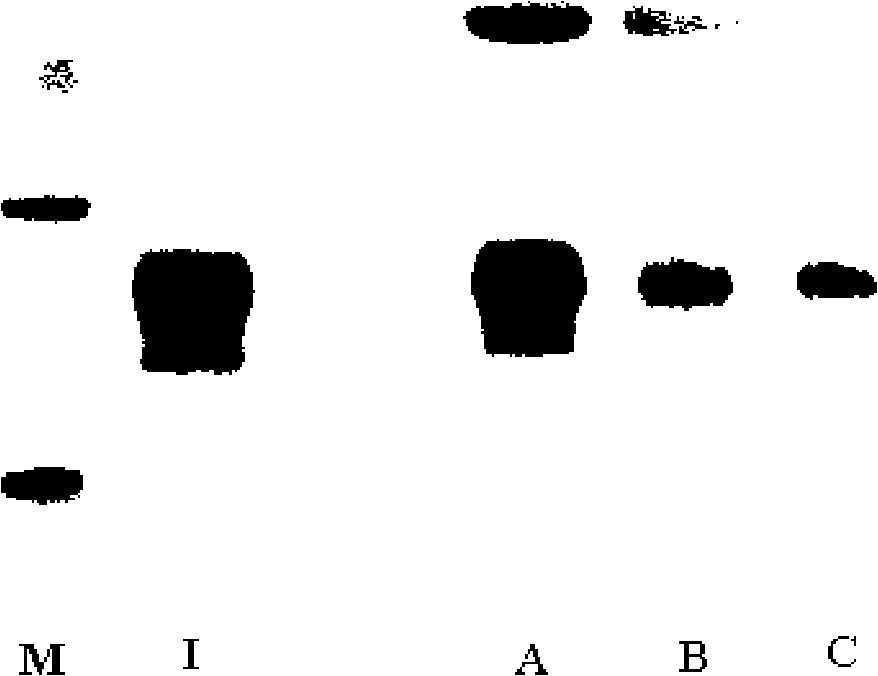
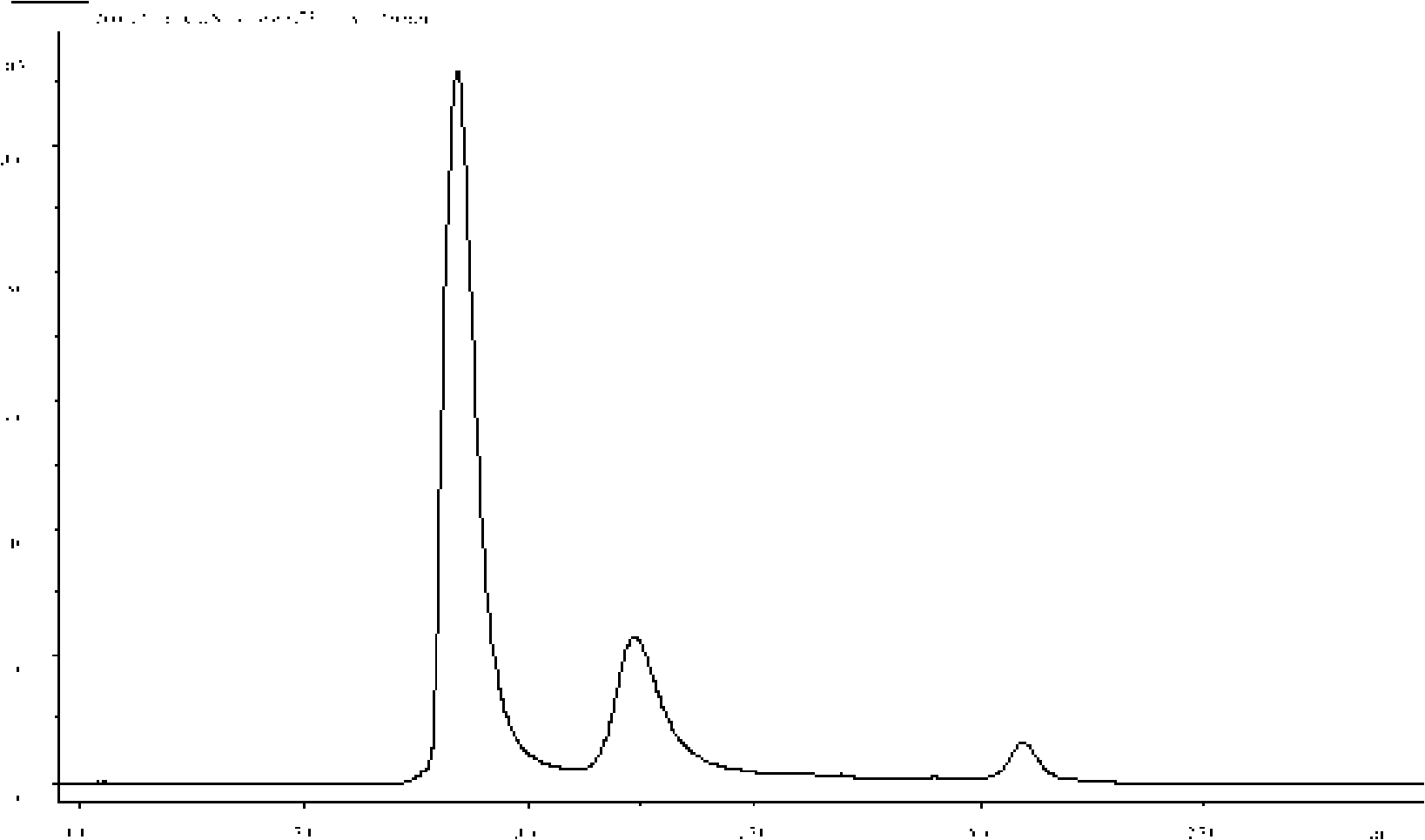
[0058] The purity of the recombinant human interferon ω used in this example is greater than 98% and maintains high biological activity; the recombinant human interferon is dissolved in 100 mM acetic acid-sodium acetate buffer (pH 4.0), and press Add monomethoxy polyethylene glycol propionaldehyde (mPEG-propionaldehyde, mPEG-ALD) in the molar ratio of recombinant human interferon ω to polyethylene glycol propionaldehyde 1:1, stir to dissolve, then add NaCNBH3, and react at room temperature 48 hours.
Embodiment 2
[0060] The purity of the recombinant human interferon ω used in this example is greater than 98% and maintains high biological activity; the recombinant human interferon is dissolved in 100 mM acetic acid-sodium acetate buffer (pH 4.0), and press Add monomethoxy polyethylene glycol propionaldehyde (mPEG-propionaldehyde, mPEG-ALD) in the ratio of 1:3 molar ratio of recombinant human interferon ω to polyethylene glycol propionaldehyde, stir to dissolve, then add NaCNBH3, and react at room temperature 24 hours.
Embodiment 3
[0062] The purity of the recombinant human interferon ω used in this example is greater than 98% and maintains high biological activity; the recombinant human interferon is dissolved in 100 mM acetic acid-sodium acetate buffer (pH 4.0), and press Add monomethoxy polyethylene glycol propionaldehyde (mPEG-propionaldehyde, mPEG-ALD) in the molar ratio of recombinant human interferon ω to polyethylene glycol propionaldehyde 1:5, stir to dissolve, then add NaCNBH3, and react at room temperature 12 hours.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com