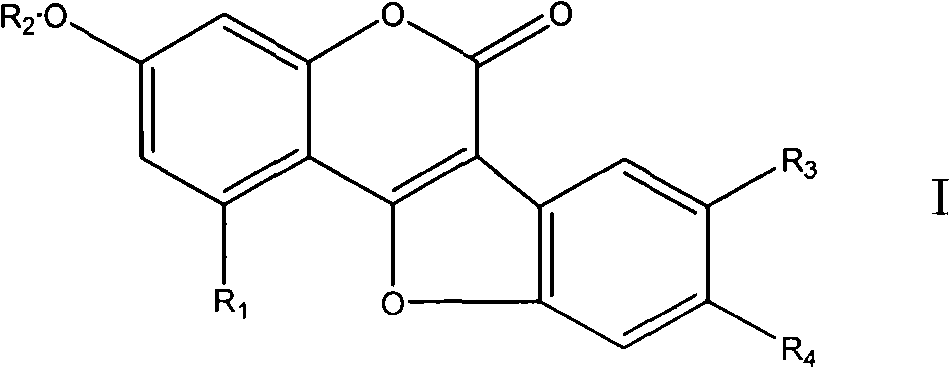
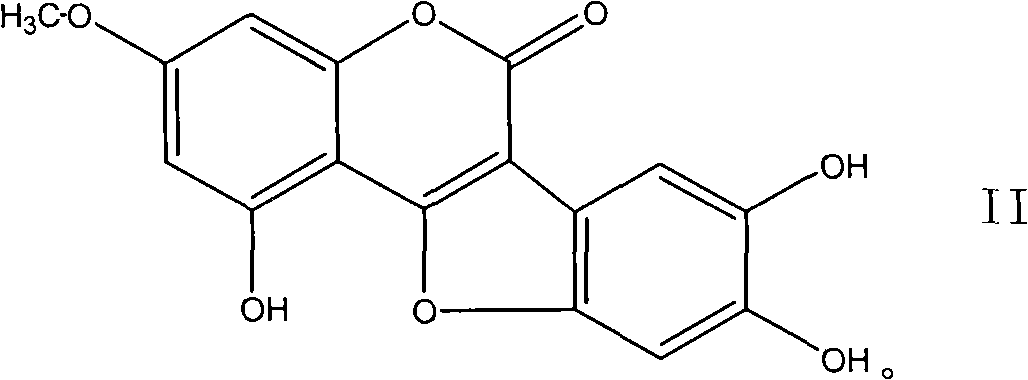
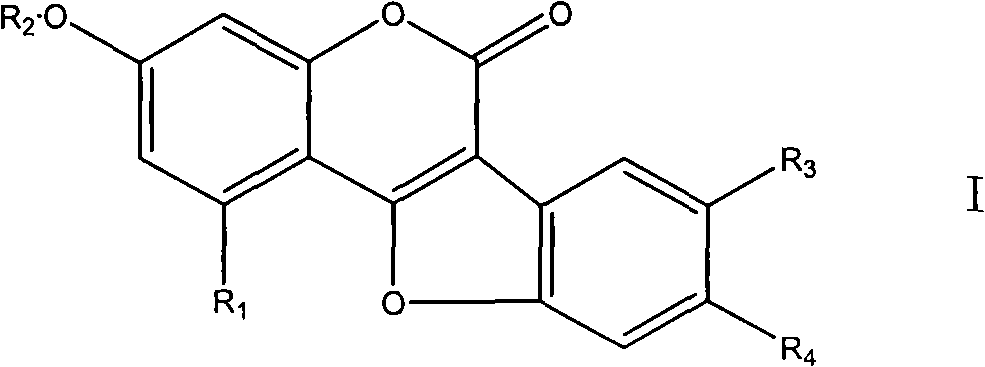
Coumarin ether compounds and new use of composition
A technology of coumarin and compounds, applied in the field of pharmacy, can solve the problems of mitochondrial toxicity, easy drug resistance, and too expensive interferon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] The compound wedelolide extracted from Eclipta alba
[0074] (1) soaking and filtering
[0075] Eclipta whole grass 300kg, fully immersed in 0.75 tons of ethanol (concentration 95%)→soak overnight (10 hours)→coarse filtration, thereby removing the whole grass residue (retention)→net filtration (suction filtration) or high-speed centrifugation (10000rpm, 10 minutes), thereby removing dust and fine residue → green clarified filtrate.
[0077] Recover ethanol by distillation, the temperature does not exceed 60°C → distill for 2 hours each time, remove the extract in the reaction kettle to the collection bucket (the extract is dark dark green, slightly viscous) → repeat the above steps until all ethanol is recovered .
[0078] (3) Second soaking and back distillation
[0079] Recover 0.75 tons of ethanol, re-soak the whole herb residue → soak overnight, the requirements for coarse filtration, net filtration and distillation recovery are the...
Embodiment 2
[0092] Inhibitory effect of wedelolide on virus in vitro
[0093] Cells and viruses: herpes simplex virus type 1 (HSV-I), respiratory syncytial virus (RSV), poliovirus type 1 (Poliovirus-1), coxsackievirus type A-7 (CVA-7), ECHO-11 and Vero cells were purchased from the National Institute for the Control of Pharmaceutical and Biological Products, Ministry of Health.
[0094] Experimental drug: APL-1 (prepared in Example 1).
[0095] Experimental method: Vero cells were inoculated in 96-well cell culture plates, the number of cells was 25,000 / well, conventionally cultured with PRMI1640 medium for 24 hours, and 100TCID were added 50 For HSV-I, RSV, Poliovirus-1, CVA-7, ECHO-11, set 6 replicate wells for each virus, discard the virus solution after 2 hours and wash with Hanks solution for 3 times, then add the concentration of 1 μg / ml, 5 μg / ml, 10 μg / ml of APL-1, incubated at 37°C for 96 hours, and observed the degree of cytopathic changes in the cells of each group.
[0096]...
Embodiment 3
[0107] Antiviral effect of wedelolide in mice
[0108] Experimental animals: 70 male SPF grade BALB / C mice, purchased from Shanghai Slack Experimental Animal Co., Ltd., weighing 18-20 g. Raised in SPF grade animal room, 12h light / 12h dark, free intake of feed and water.
[0109] Virus strain: herpes simplex virus type I (HSV-1) Sm44 strain, purchased from National Institute for the Control of Pharmaceutical and Biological Products, Ministry of Health.
[0110] Experimental drugs: APL-1 (prepared in Example 1), ribavirin (ribavirin, purchased from Jiaozuo Kangli Pharmaceutical Co., Ltd., 0.1 g / ml).
[0111] Experimental method: Using different dilutions of the virus liquid, the experimental mice were inoculated intracranially with HSV-1 (TCID 50 =10 -7 ·ml -1 ), observed for 14 days. According to the number of mouse deaths, calculate the LD of the virus 50 , to determine the amount of virus inoculum. According to the probability unit method of half number effect, measure...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com