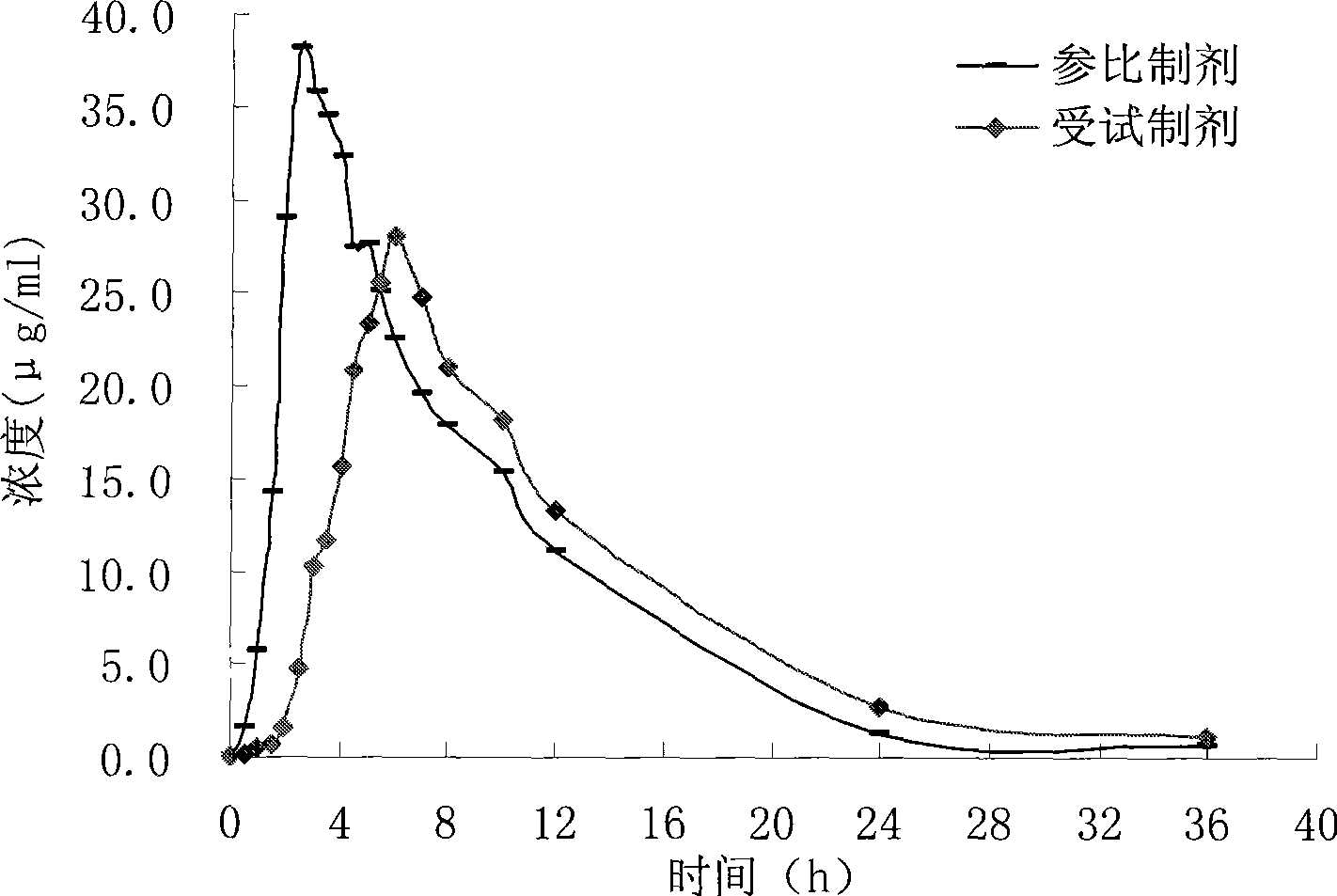
Cefixime sustained-release tablets and preparation method thereof
A technology of cefixime and sustained-release materials, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve the difficulty of industrialization and the cumbersome preparation process of cefixime sustained-release tablets And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
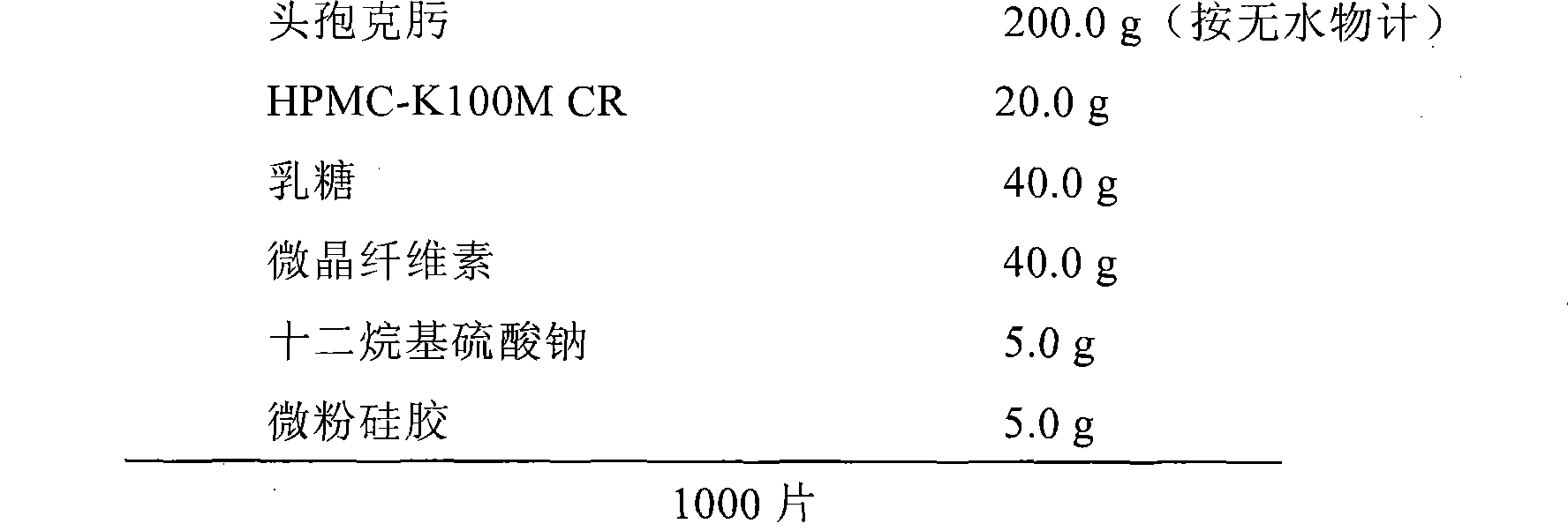
Embodiment 1
[0026]
[0027] Preparation method: cefixime, HPMC-K100M CR, lactose, and microcrystalline cellulose are passed through a 40-mesh sieve, and sodium lauryl sulfate and micropowder silica gel are passed through a 80-mesh sieve. Take the prescribed amount of cefixime and sodium lauryl sulfate and mix evenly, add the prescribed amount of lactose, microcrystalline cellulose, HPMC-K100M CR and mix thoroughly, then add the prescribed amount of micropowder silica gel, mix evenly, and press into tablets.
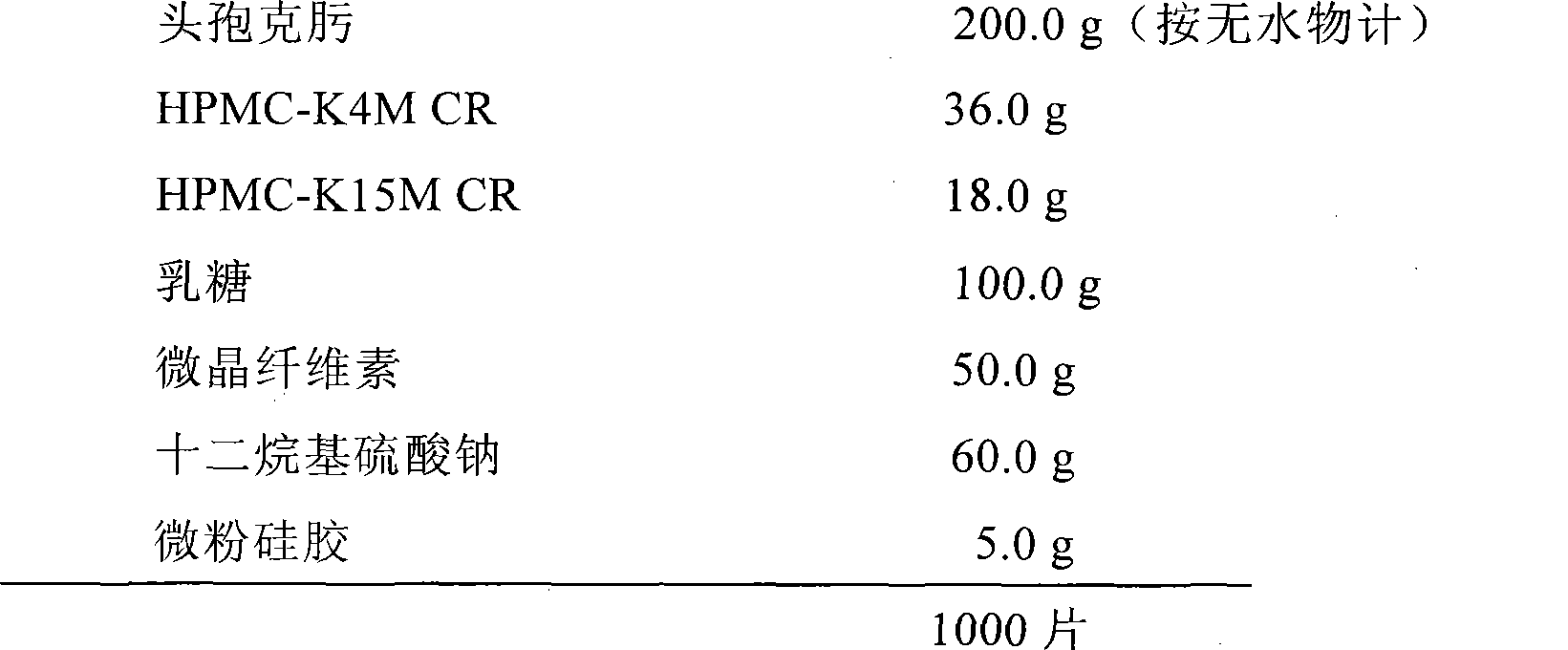
Embodiment 2
[0029]
[0030] Preparation method: replace HPMC-K100M CR in Example 1 with HPMC-K4M CR and HPMC-K15M CR, and the rest are the same as Example 1.
Embodiment 3
[0032]
[0033] Preparation method: take cefixime, HPMC K4M CR, microcrystalline cellulose, and lactose and pass through a 40-mesh sieve, and poloxamer, sodium lauryl sulfate, and micropowdered silica gel pass through a 80-mesh sieve. Weigh the prescribed amount of cefixime, sodium lauryl sulfate, and poloxamer and mix evenly, then add the prescribed amount of HPMC-K4M CR, microcrystalline cellulose, and lactose, mix thoroughly, and then add the prescribed amount of micropowder silica gel to mix Uniformly, pressed into tablets, that is to say.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity range | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com