Medical devices having nanoporous coatings for controlled therapeutic agent delivery
a technology of controlled drug delivery and medical devices, applied in the direction of prosthesis, therapy, blood vessels, etc., can solve the problems of limited amount of drug loaded, device function beyond drug delivery,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
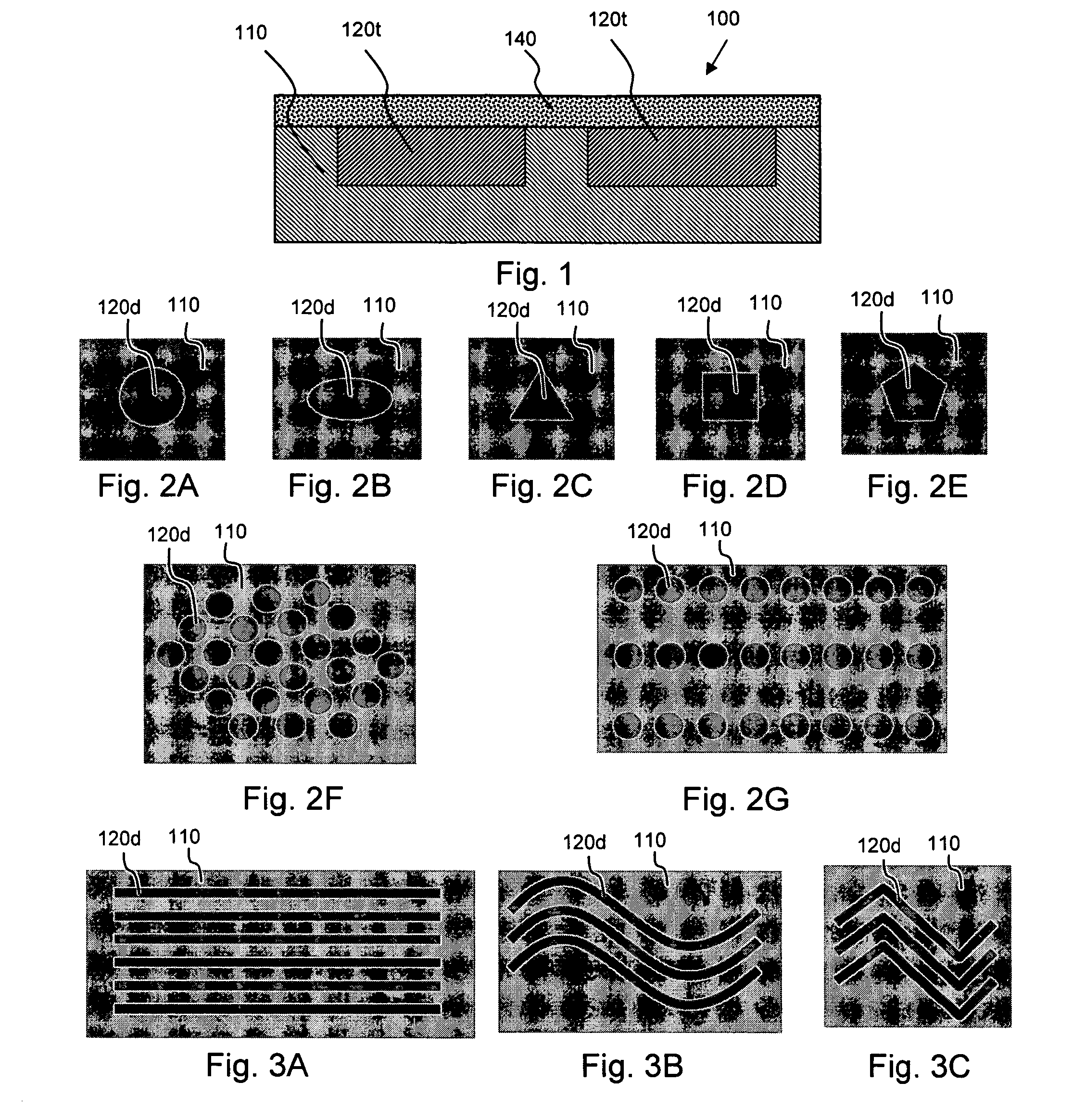
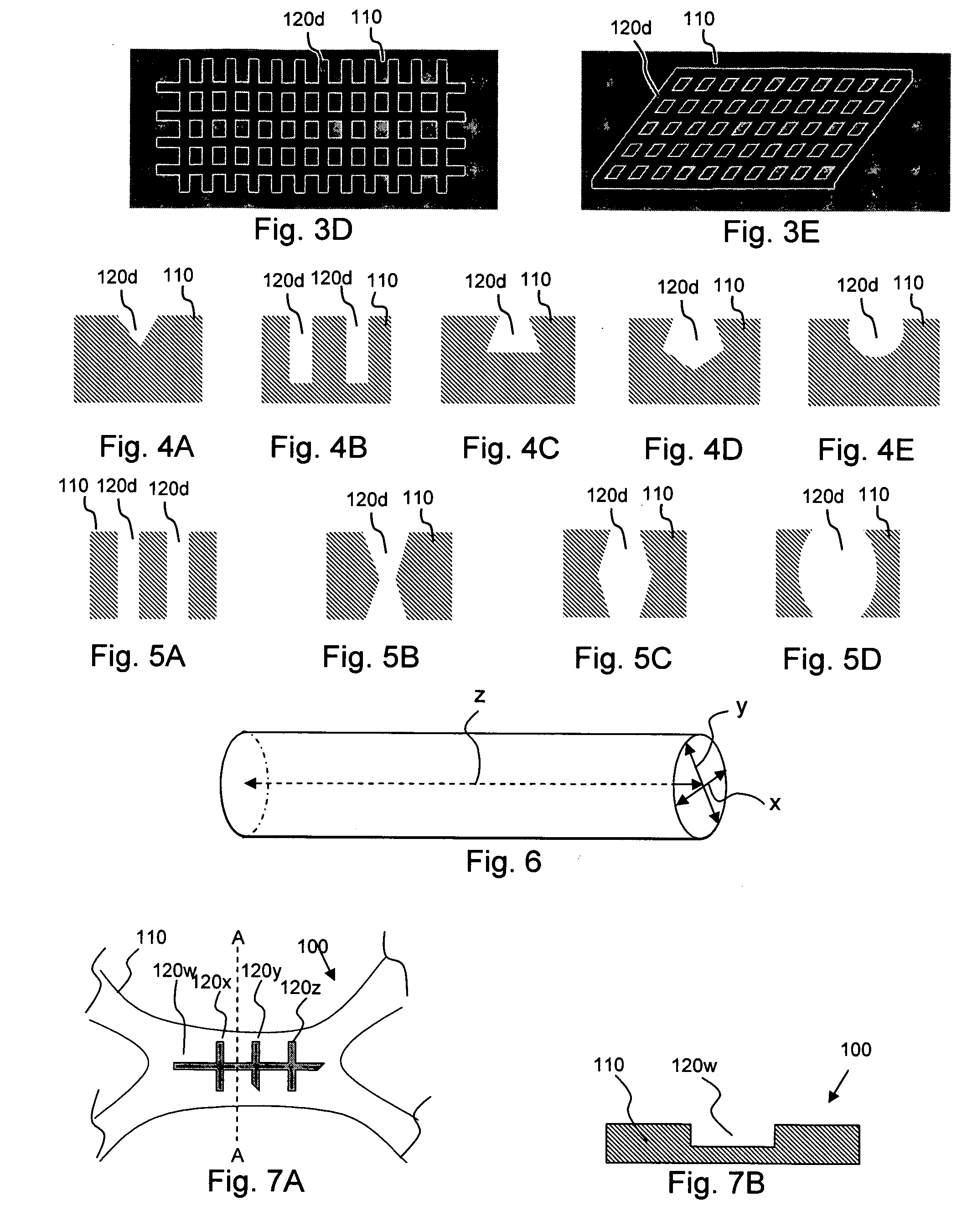
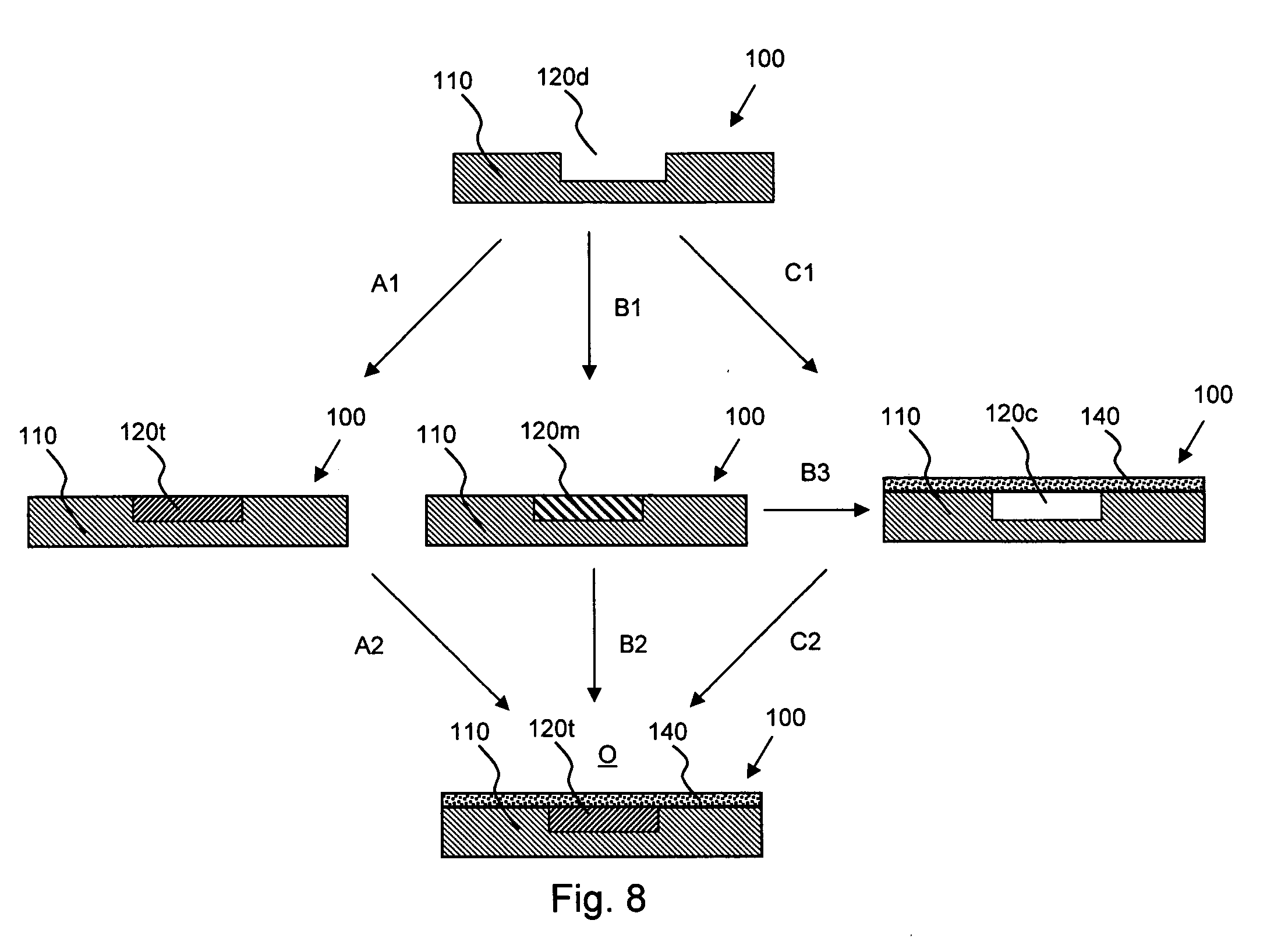
[0020] According to an aspect of the invention, medical devices are provided which contain (a) one or more depressions that contain at least one therapeutic agent, and (b) one or more nanoporous regions, disposed over the therapeutic-agent-containing depressions, which regulate transport of species between the therapeutic-agent-containing depressions and the exterior of the device.
[0021] For example, a therapeutic agent may be transported from the therapeutic-agent-containing depressions such that it is released in vivo, an in vivo species may be transported into the therapeutic-agent-containing depressions where it reacts with the therapeutic agent to form another species (e.g., a less detrimental or more beneficial species) which is then transported from the depressions, and so forth.
[0022] The implantable or insertable medical devices of the invention are also configured to provide a therapeutic function beyond species transport, for instance, providing mechanical, thermal, mag...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com