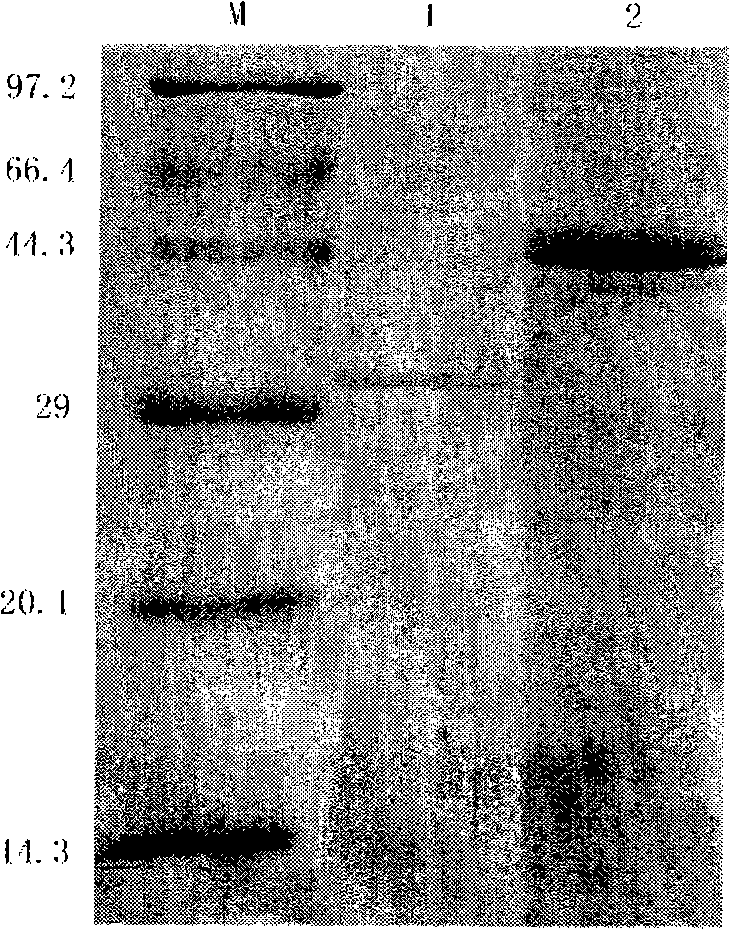
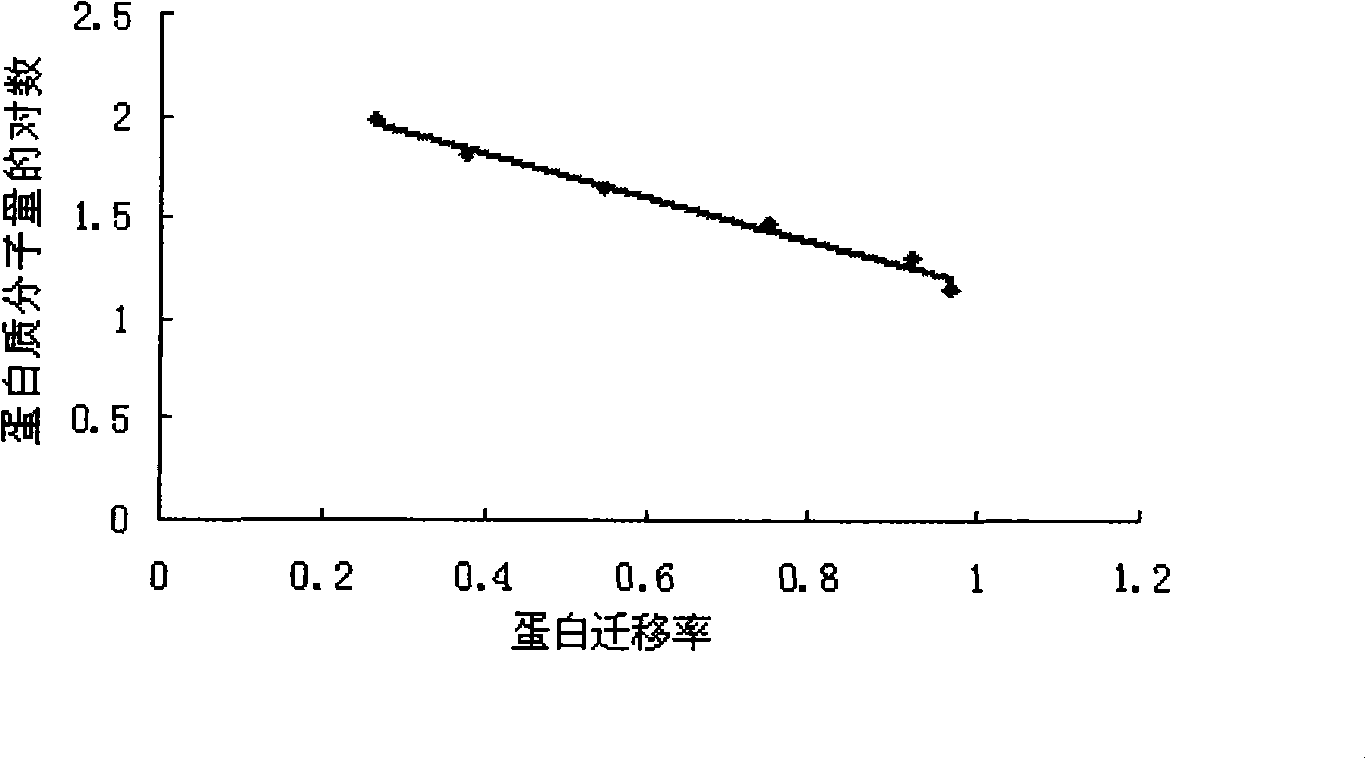
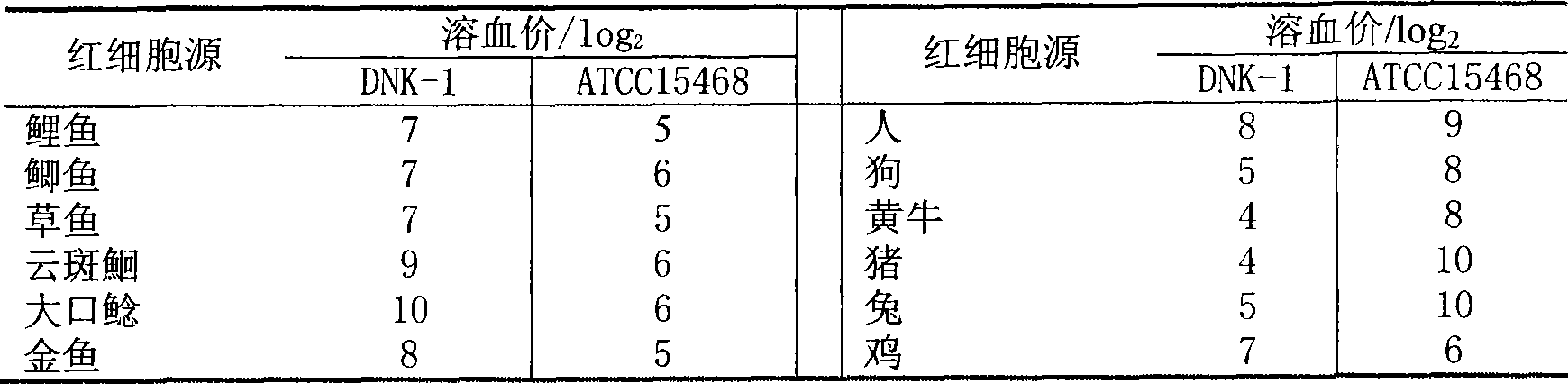
Vaccine for fish and application
A vaccine composition and application technology, applied in skin diseases, bacterial antigen components, antibacterial drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0145] Embodiment 1 Preparation of whole bacteria vaccine of the present invention
[0146] 1. Materials and methods
[0147] 1.1 Materials
[0148] Test bacteria: Aeromonas caviae (A.caviae) isolated and identified from diseased largemouth catfish
[0149] Experimental fish: purchased from the Ya'an market, healthy, disease-free and injury-free, weighing about 200g, and kept for 7 days before the experiment.
[0150] Main reagents: Azone (chemically pure, produced by Chengdu Kelong Chemical Reagent Factory); racemic anisodamine hydrochloride injection (1ml: 10mg, 05072531, produced by Tianjin Pharmaceutical Jiaozuo Co., Ltd.). Tryptone, soybean peptone, nutrient agar, Beijing Boaoxing Biotechnology Co., Ltd.
[0151] Medium: TSA and TSB
[0152] 1.2 Method
[0153] 1.2.1 Feeding management
[0154] It is raised in an aquarium and continuously increases oxygen for 24 hours; the water source is tap water after aeration and dechlorination treatment, its pH value is 6.8-7.2...
Embodiment 2
[0183] Embodiment 2 The preparation and application method of vaccine preparation (aqueous) of the present invention
[0184] [Preparation of the vaccine]: Aeromonas caviae (DKN-1) was resuscitated and cultured, then primary cultured in TSB broth, then secondary cultured in TSA and enlarged cultured in TSB broth. Marin 28°C, 110r / min, shaking inactivation for 36h, after the inactivation is complete after inspection, aseptically bottled and ready for use. When used in combination with 10mg / l azone penetration enhancer, they can be mixed together or administered separately at the same time.
[0185] [Composition and shape]: This strain is an inactivated whole bacterin vaccine for southern largemouth catfish ulcer syndrome. The vaccine should be brownish-yellow, with white precipitate on standing.
[0186] [Specification]: The specification of this product is 500mL per bottle, and under normal circumstances, the bacteria content per milliliter is 10 10 Above CFU, when it canno...
Embodiment 3
[0196] Embodiment 3 The preparation and application method of the vaccine preparation (powder) of the present invention
[0197] 1. Vaccine Preparation
[0198] (1) Bacterial recovery
[0199] Under sterile conditions, take the strains stored at 4°C and use an inoculation loop to pick a small amount of bacteria from the strain tube to inoculate into 10ml TSB broth, put the inoculated broth into a water bath shaker, set at 28°C, 110r / min, shaking culture for 24h.
[0200] (2) Proliferation and culture of bacteria liquid
[0201] ①Primary training
[0202] Inoculate 10ml of the revived cultured bacterial solution and inoculate 500ml TSB broth again, put the inoculated broth into a water bath shaker, 28°C, 110r / min, and shake for 24h.
[0203] ②Secondary culture of bacterial liquid
[0204] Spread the secondary cultured TSB broth evenly on the prepared ordinary nutrient agar plate, put the coated ordinary plate into a constant temperature incubator at 28°C, and cultivate for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Relative molecular weight | aaaaa | aaaaa |
| Relative molecular weight | aaaaa | aaaaa |
| Relative molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com