Novel disulfonic acid type alkyl imidazole ionic liquid, preparation and uses thereof
A technology of alkylimidazole and ionic liquid, which is applied in the field of new bissulfonic acid acidic ionic liquid and its preparation, can solve the problems that the reaction conditions are not very friendly to the environment and cannot be surpassed, and achieve high reaction yield and low dosage , good catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
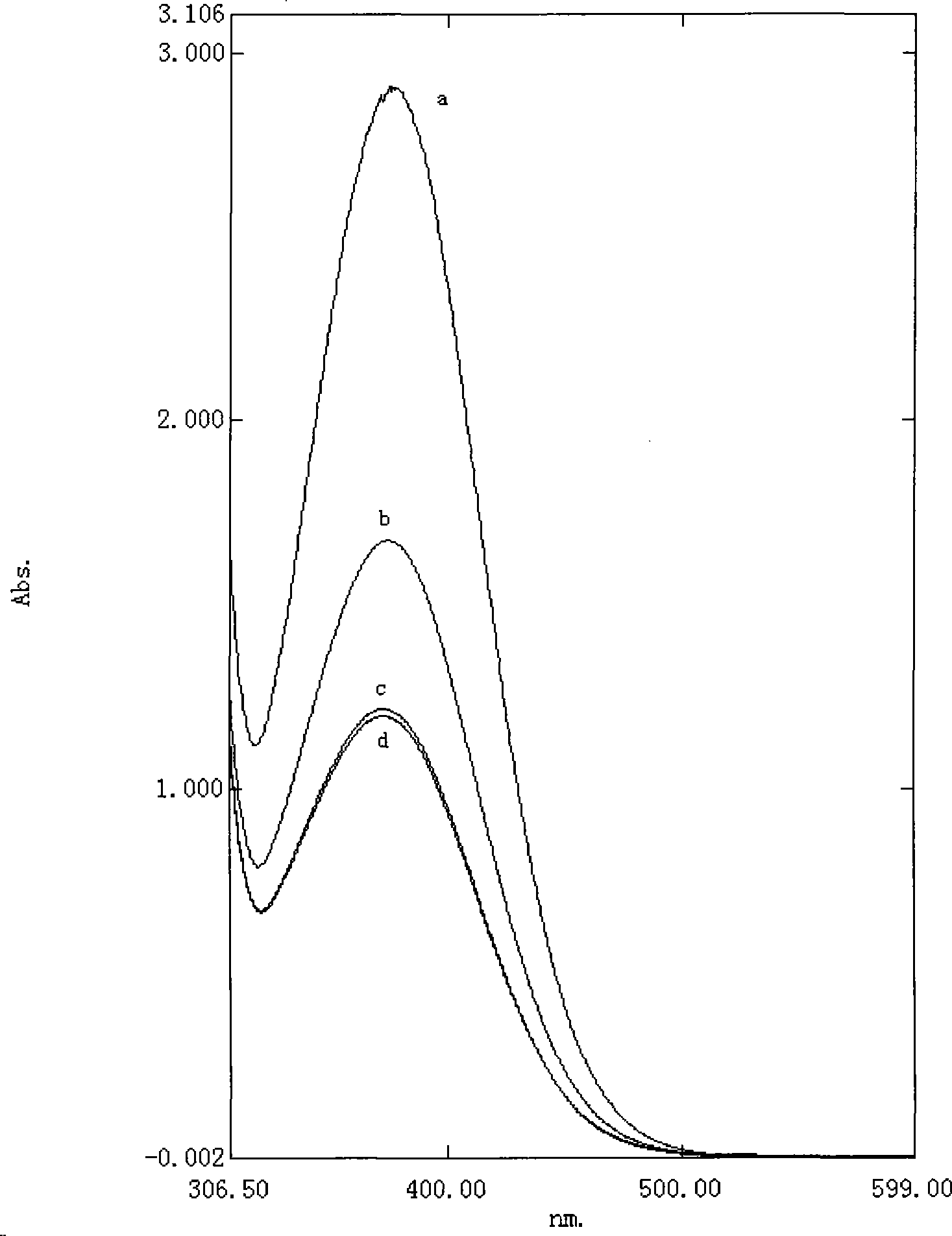
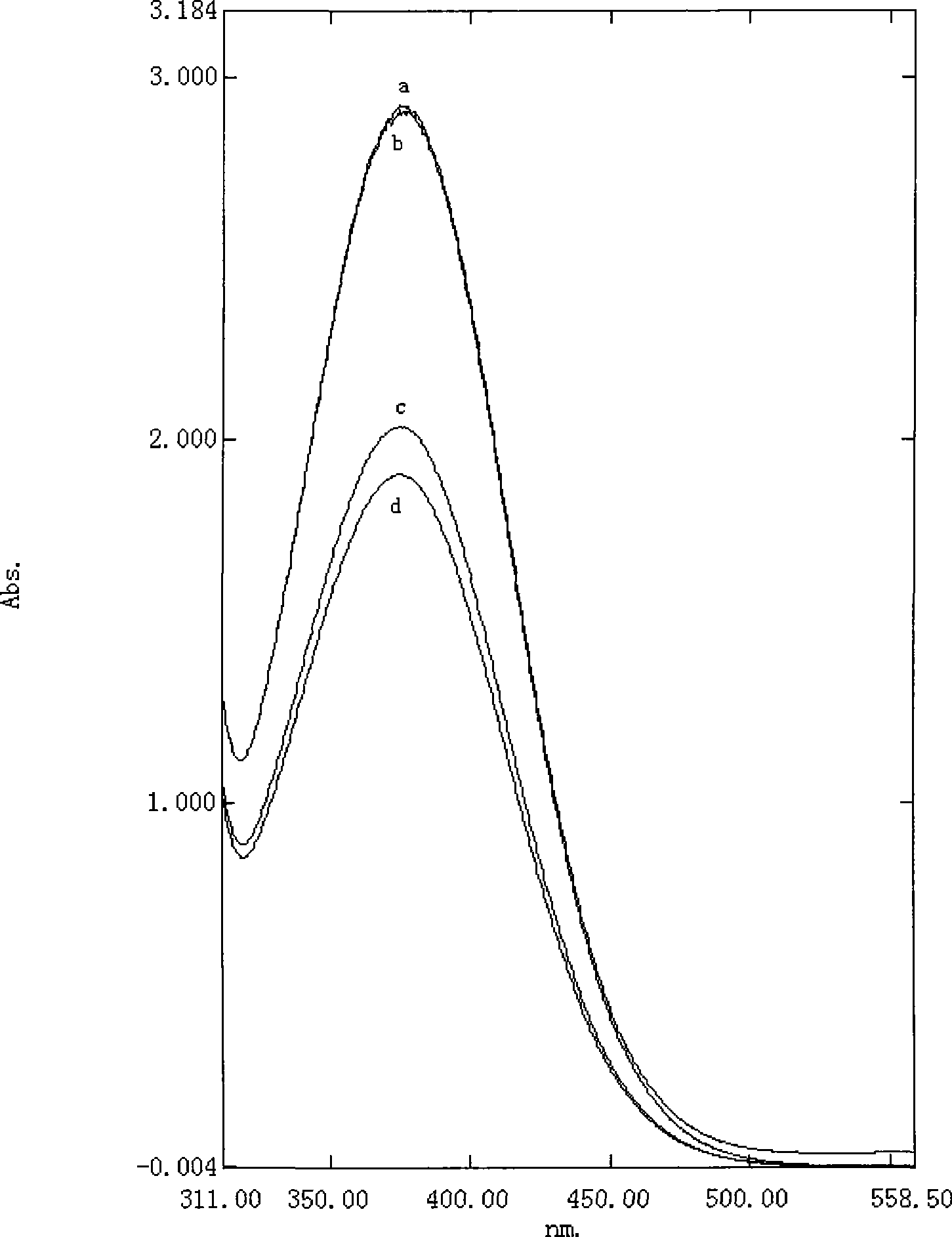
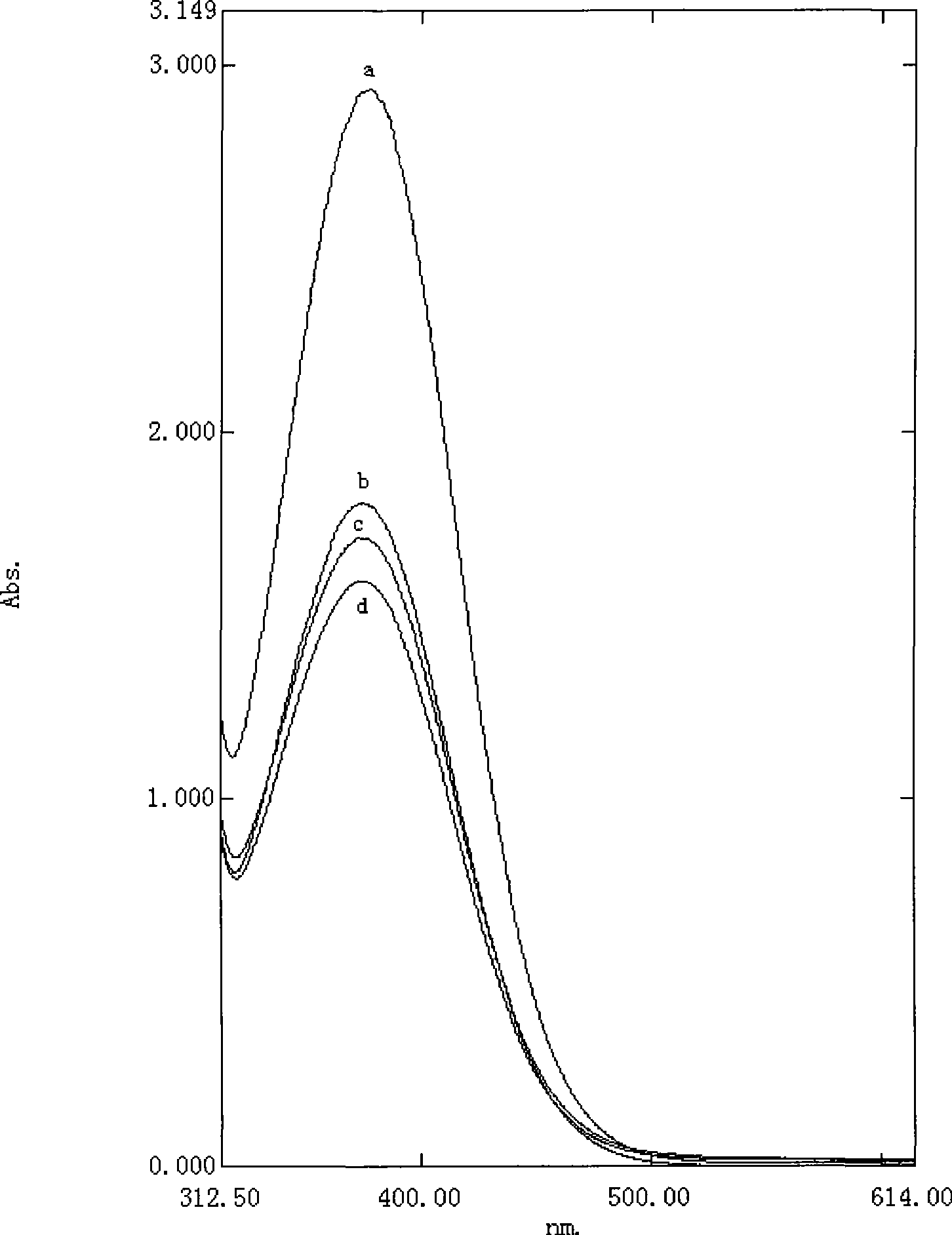
Image
Examples
Embodiment 1
[0061] [(HSO 3 -p) 2 im][HSO 4 ] (n=1, Y=HSO 4 )Synthesis:
[0062] Trimethylsilimidazole (14.0g0.1mol) was added to a 100mL three-necked flask, and 1,3-propane sultone (24.4g0.2mol) was slowly added dropwise under ice-bath conditions. Stirring was continued for half an hour under ice-bath conditions, about 1.8 mL (0.1 mol) of water was added dropwise, stirring was continued for 2 hours, and water and trimethylsilanol were distilled off under reduced pressure to obtain an intermediate zwitterionic compound. Add sulfuric acid (10.0g0.1mol) dropwise to the about 2mol / L aqueous solution of the obtained zwitterionic compound, reflux for 8 hours after the dropwise addition, and finally remove the solvent water by precipitation under reduced pressure at 90°C to obtain a light yellow transparent ion Liquid 38.9g, yield 95%.
Embodiment 2
[0064] [(HSO 3 -p) 2 im][HSO 4 ] (n=1, Y=HSO 4 )Synthesis:
[0065] Trimethylsilimidazole (14.0g0.1mol) was added to a 100mL three-necked flask, and 1,3-propane sultone (24.4g0.19mol) was slowly added dropwise under ice-bath conditions. Stirring was continued for half an hour under ice-bath conditions, about 1.8 mL (0.1 mol) of water was added dropwise, stirring was continued for 2 hours, and water and trimethylsilanol were distilled off under reduced pressure to obtain an intermediate zwitterionic compound. Add sulfuric acid (9.0g0.09mol) dropwise to the about 2mol / L aqueous solution of the obtained zwitterionic compound, reflux for 8 hours after the dropwise addition, and finally remove the solvent water by precipitation under reduced pressure at 80°C to obtain a light yellow transparent ion Liquid 32.76g, yield 80%.
Embodiment 3
[0067] [(HSO 3 -b) 2 im][HSO 4 ] (n=2, Y=HSO 4 )Synthesis
[0068] Add trimethylsilimidazole (14.0g0.1mol) into a 100ml three-necked flask, and slowly add 1,4-butyl sultone (27.2g0.2mol) dropwise under ice-bath conditions. Stirring was continued for half an hour under ice-bath conditions, about 1.8ml (0.1mol) of deionized water was added dropwise, stirring was continued for 2h, and water and trimethylsilanol were distilled off under reduced pressure to obtain an intermediate zwitterionic compound. Add 50ml of deionized water to the obtained zwitterionic compound and stir evenly, and add sulfuric acid (10.0g0.1mol) dropwise. After the dropwise addition, heat and reflux at 90°C for 6 hours, and finally remove it by precipitation under reduced pressure at 90°C. Solvent water to obtain 40.7 g of light yellow transparent ionic liquid with a yield of 94%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com