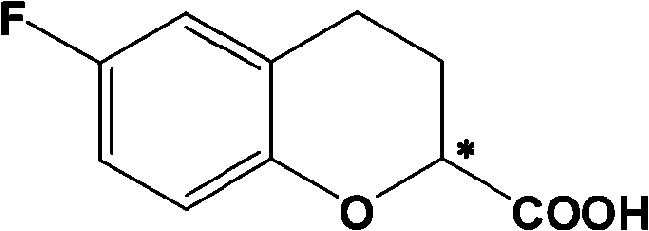
Method for preparing optical pure 6-Fluoro-3,4-dihydro-2H-1-benzopyran-2-carboxylic acid
A benzopyran and optical technology, applied in the field of cardiovascular drugs, can solve the problems of high cost, complicated operation of the splitting method, low total yield and the like, and achieve the effects of simple method, high yield and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
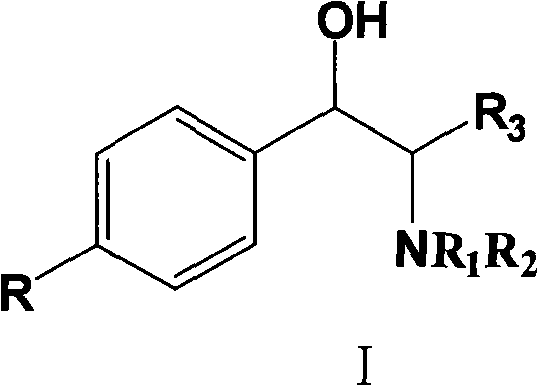
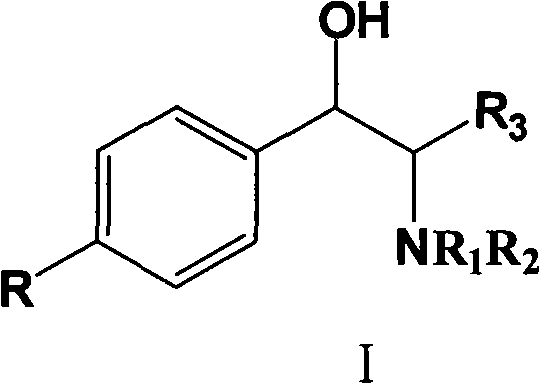
[0031] 0.98 g of 6-fluoro-3,4-dihydro-2H-1-chromene-2-carboxylic acid, 1.2 g of optically pure (1R,2S)-N,N-dimethyl 1-p-nitro Add phenyl-2-amino-1,3-propanediolamine and 15mL ethanol into a 25mL round bottom flask, heat to reflux until all solids are dissolved, stir for 5 minutes, slowly lower to 30°C, and filter to obtain diastereoisomeric The solid 0.9 g of body salt, wherein (S) configuration acid is the majority, e.e. value is 89%, and the yield is 41%.
Embodiment 2
[0033] 9.8 grams of 6-fluoro-3,4-dihydro-2H-1-chromene-2-carboxylic acid, 12 grams of optically pure (1R,2S)-N,N-dimethyl 1-p-nitro Phenyl-2-amino-1,3-propanediol and 130mL ethanol were added to a 250mL round bottom flask, heated to reflux until all the solids were dissolved, stirred for 5 minutes, slowly lowered to 30°C, and filtered to obtain 10 grams of solids, wherein ( The acid in the S) configuration was in the majority, the e.e. value was 84%, and the yield was 46%.
[0034] The obtained solid was recrystallized once with 39 mL of ethanol to obtain 8.2 g of solid, which was dissociated with 50 mL of 1N hydrochloric acid, and then extracted with dichloromethane (100 mL×3). The dichloromethane layers were combined, dried and concentrated to obtain (S)- (+)-6-Fluoro-3,4-dihydro-2H-1-chromene-2-carboxylic acid 3.65 g, e.e. value 100%, yield 37%.
Embodiment 3
[0036] 58.8 grams of 6-fluoro-3,4-dihydro-2H-1-chromene-2-carboxylic acid, 72 grams of optically pure (1R,2S)-N,N-dimethyl 1-p-nitro Phenyl-2-amino-1,3-propanediol and 700mL of ethanol were added to a 1000mL round-bottomed flask, heated to reflux until all the solids were dissolved, stirred for 5 minutes, slowly lowered to 30°C, and filtered to obtain 76 grams of solids, (S ) configuration acid is the majority, e.e. value is 47%, and the yield is 58%.
[0037]The obtained solid was recrystallized once with 370 mL of ethanol to obtain 49.7 grams of solid, which was dissociated with 200 mL of 1N hydrochloric acid, and then extracted with dichloromethane (300 mL×3). The dichloromethane layers were combined, dried and concentrated to obtain (S)- (+)-6-Fluoro-3,4-dihydro-2H-1-chromene-2-carboxylic acid 22.1 g, e.e. value 99%, yield 37.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com