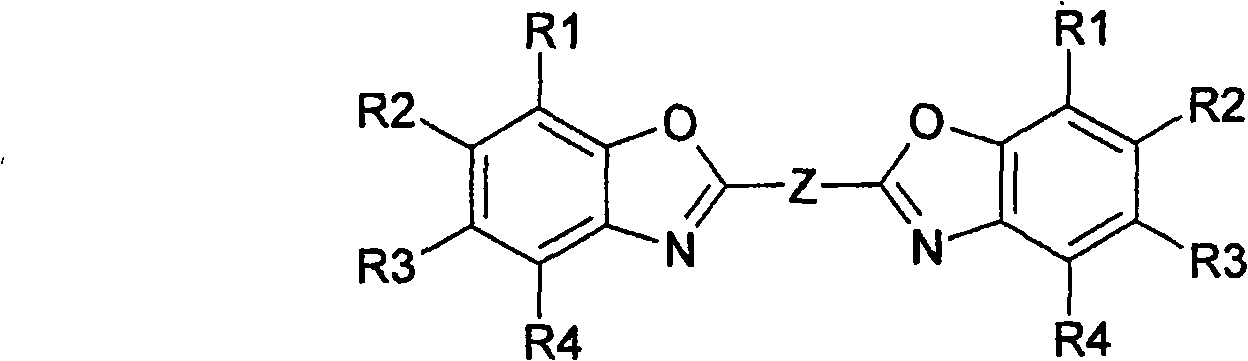
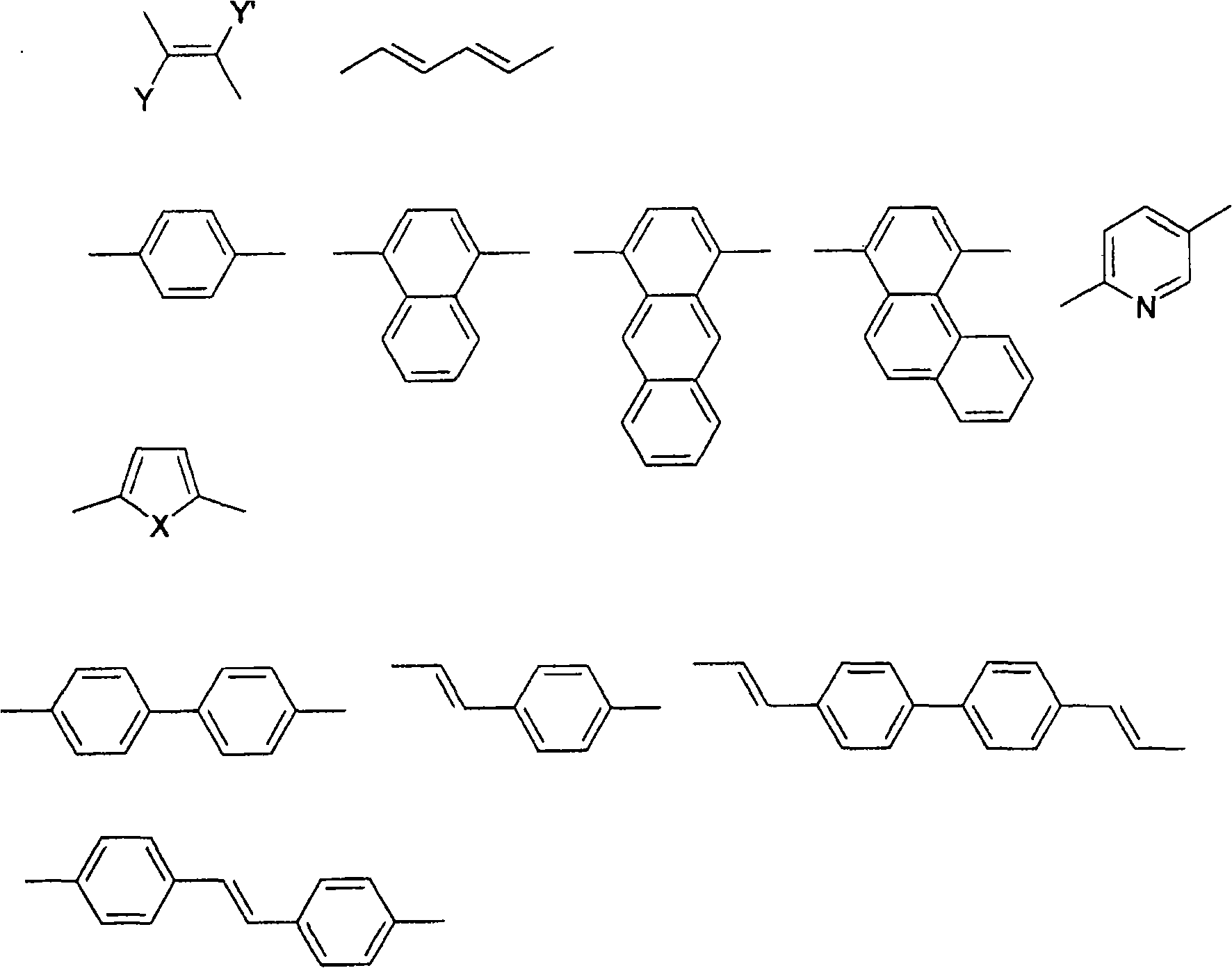
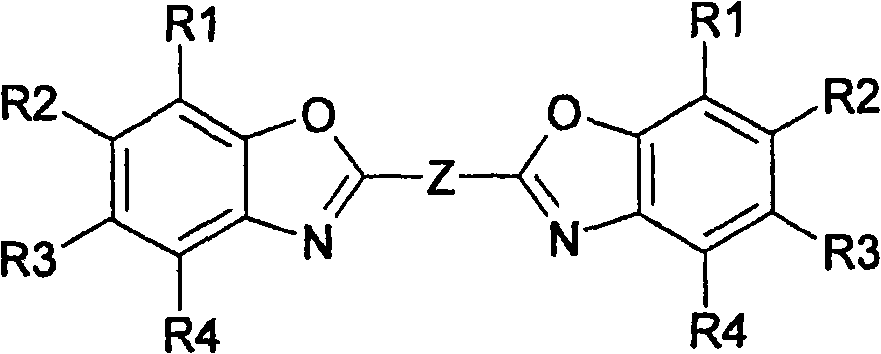
Method for producing bisbenzoxazoles
A technology of benzoxazole and conjugated double bond, which is applied in the field of preparing bisbenzoxazole and can solve problems such as undesired effects and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1: Preparation of 1,4-bis-(benzoxazol-2'-yl)-naphthalene
[0062] Suspend 3.55 g (16.4 mmol) of naphthalene-1,4-dicarboxylic acid and 4.05 g (37.1 mmol) of o-aminophenol in 12.1 g of 1,2,3,4-tetralin and stir under argon Heat to 180°C for 20 minutes. The ammonium salt-containing suspension thus obtained was admixed with 0.35 g of boric acid and 0.1 g of p-toluenesulfonic acid and exposed to microwave irradiation at 300 W for 2 hours in a vessel with KPG-stirrer and water trap. A temperature of about 230° C. measured by means of an IR sensor was reached. This temperature is kept constant by evaporative cooling. The reaction mixture was then cooled to room temperature within 10 minutes, where the product crystallized out in the form of yellow needles.
[0063] HPLC of the reaction batch mixture showed complete conversion of naphthalene-1,4-dicarboxylic acid to 1,4-bis-(2"-benzoxazolyl)-naphthalene. After filtration, the crystals were washed with methanol and dr...
Embodiment 2
[0063] HPLC of the reaction batch mixture showed complete conversion of naphthalene-1,4-dicarboxylic acid to 1,4-bis-(2"-benzoxazolyl)-naphthalene. After filtration, the crystals were washed with methanol and dried After that, 1,4-bis-(benzoxazol-2'-yl)-naphthalene with a purity of more than 99.5% was obtained. Embodiment 2: Preparation of 1,4-bis-(benzoxazol-2'-yl)-naphthalene in a closed system 2'-yl)-naphthalene
[0064] 0.71 g (3.3 mmol) of naphthalene-1,4-dicarboxylic acid and 0.81 g (7.4 mmol) of o-aminophenol were suspended in 2.4 g of N-methylpyrrolidone with stirring in a pressure-resistant glass test cell. After addition of 169 μl of tetrabutoxytitanium, the ammonium-salt-containing suspension thus prepared was exposed to microwave radiation at 300 W for 15 minutes in a pressure-resistant, closed test chamber with stirring and external cooling. A temperature of about 225[deg.] C., measured by means of an IR sensor, is reached, with the pressure rising to approximate...
Embodiment 3
[0066] Example 3: Preparation of 1,2-bis-(5-methylbenzoxazol-2'-yl)-ethene
[0067] 2.3 g of fumaric acid and 5.52 g of o-amino-p-cresol were homogenized in 12.45 g of tetralin with heating and stirring. The ammonium salt-containing suspension thus prepared was admixed with 42 mg boric acid and 12 mg p-toluenesulfonic acid and exposed to microwave irradiation at 300 W for 30 minutes with stirring in an open apparatus with complete external cooling. A temperature of about 220° C. measured by means of an IR sensor was reached. The reaction mixture was then cooled to room temperature within 10 minutes. The yield of 1,2-bis-(5-methylbenzoxazol-2'-yl)-ethylene was 65% based on fumaric acid.
[0068] After filtering off, washing the crystals with methanol, extracting the remaining acid by stirring with alcoholic sodium hydroxide solution and drying, 1,2-bis-(5-methylbenzoxazole-2' with a purity of more than 98% is obtained -yl)-ethylene.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com