Dissociating agents, formulations and methods providing enhanced solubility of fluorides
A technology of fluoride and dissociating agent, applied in the direction of aqueous electrolyte battery, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Example 1 : Electrolytes and dissociators for electrochemical cells
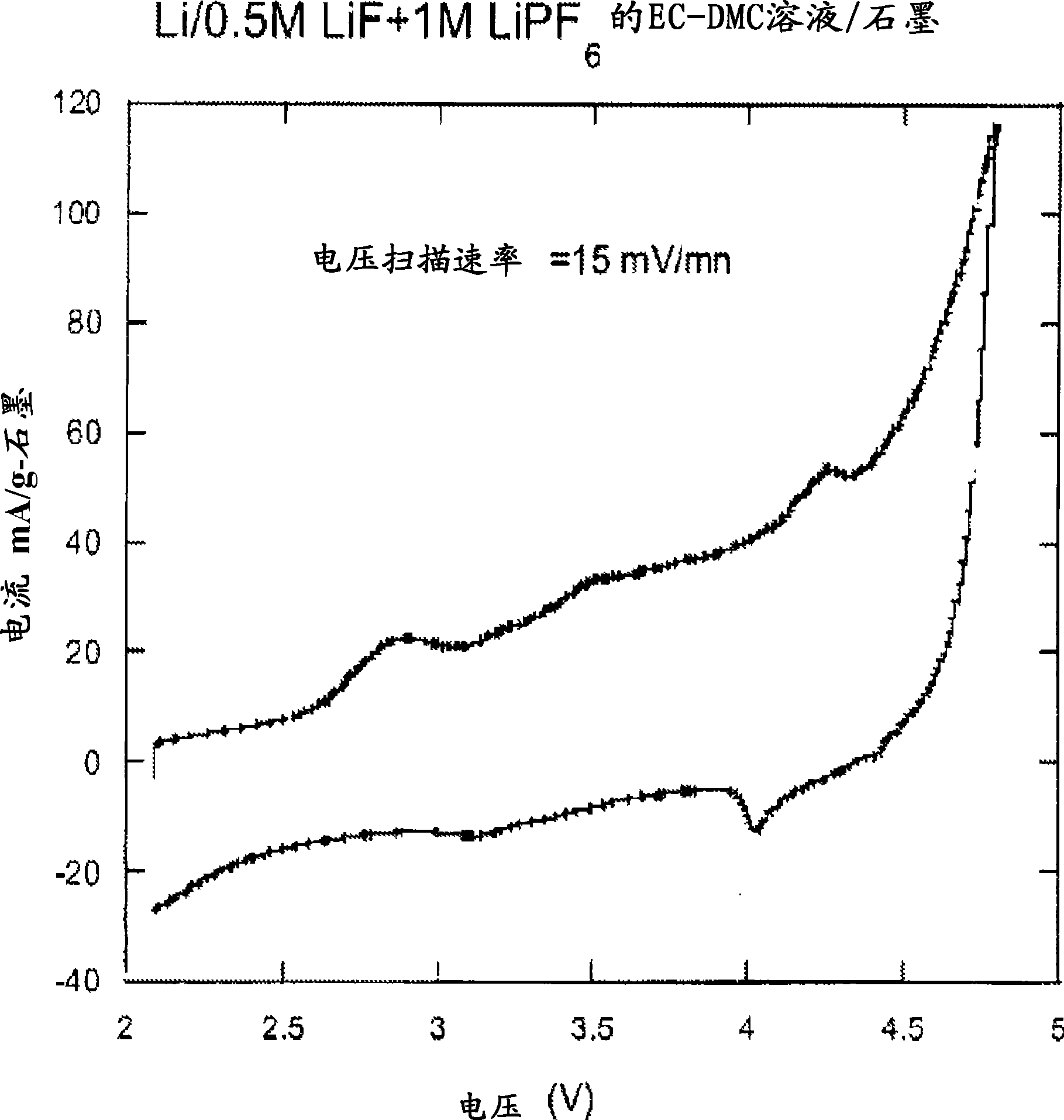
[0081] To illustrate the chemistry and use of the additives, compositions, formulations and methods of the invention, electrolytes of the invention were prepared and incorporated into lithium electrochemical cells. The electrolyte used for evaluation consisted of LiF and a suitable dissociator dissolved in a selected nonaqueous organic solvent or mixture of nonaqueous organic solvents. The electrical performance of the electrochemical cells was evaluated to demonstrate the beneficial chemical and physical properties of the electrolytes of the invention.
[0082] 1. Preparation of mother liquor : Prepare 1M LiPF in an argon-filled dry box 6 EC-DMC solution and LiBF 4 γ-BL solution and LiAsF 6 solution of PC and LiClO 4 PC solution (EC is ethylene carbonate, DMC is dimethyl carbonate, PC is propylene carbonate). These solutions are referred to as mother liquors throughout the specification.
...
Embodiment 2
[0093] Example 2: Contrast of LiF-containing and LiF-free carbon fluoride electrode lithium half cells
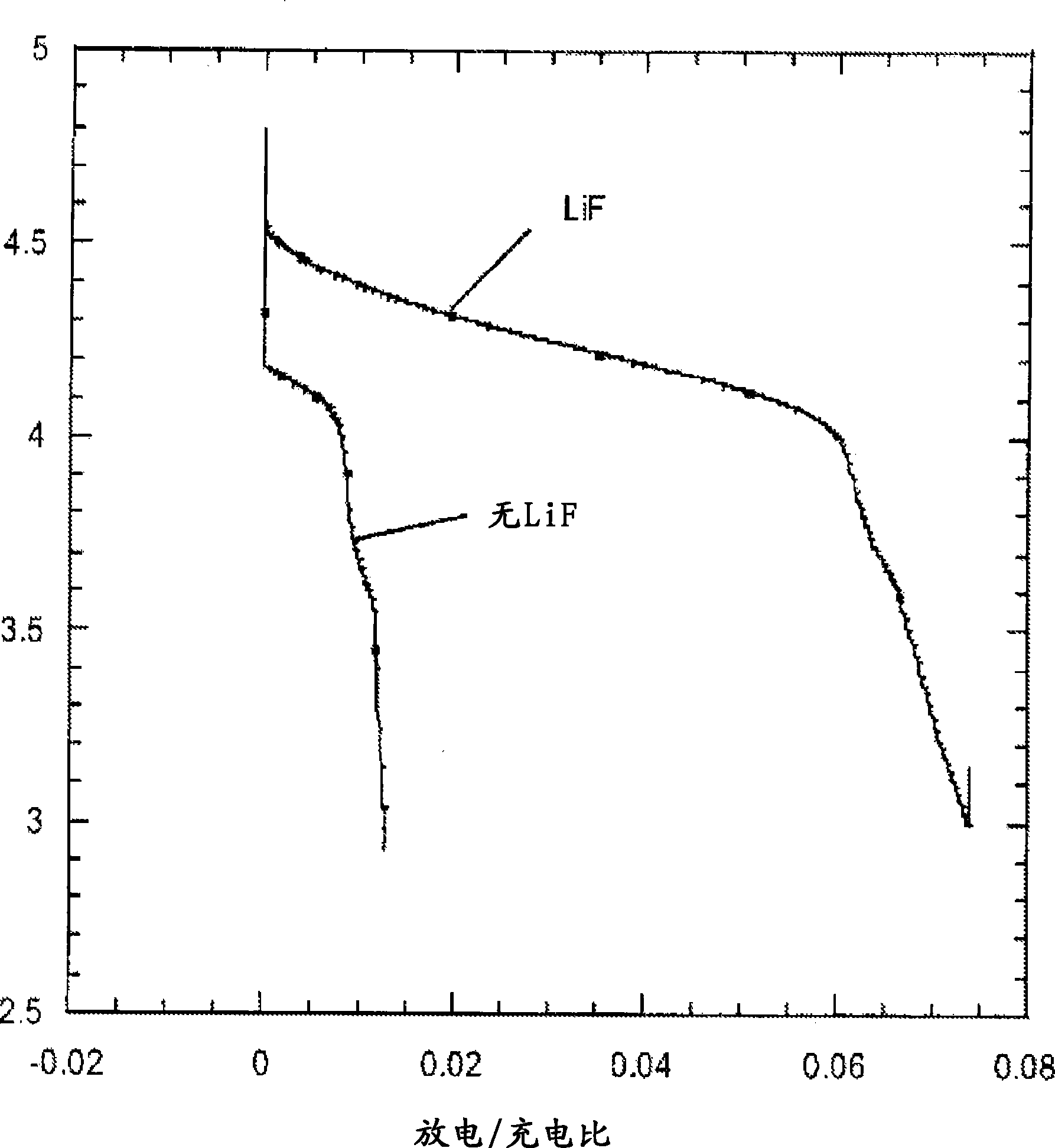
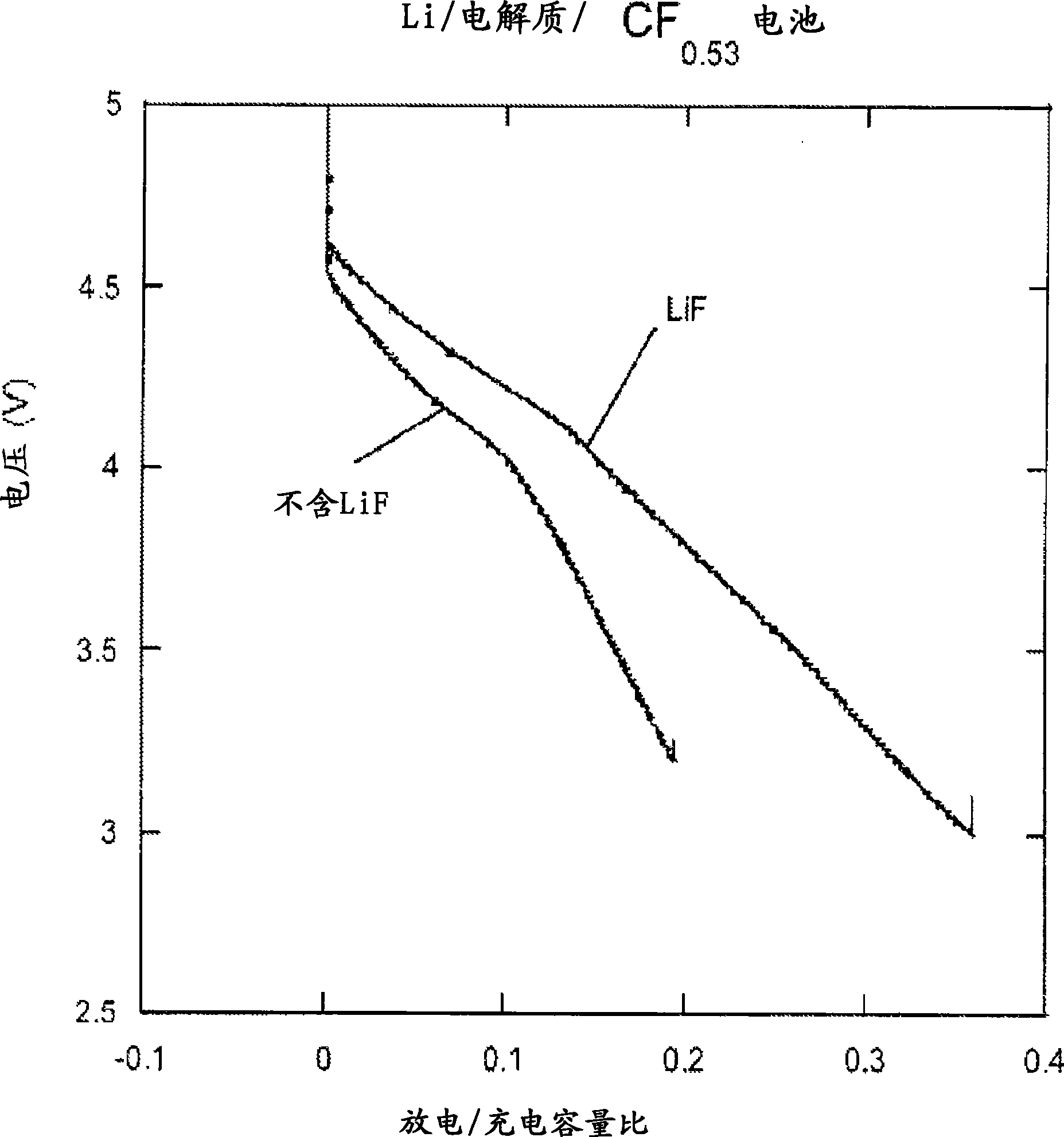
[0094] Prepare two carbon fluoride electrodes (CF 0.125 ) lithium half-cell. One battery contains 1M LiPF 6 The propylene carbonate (PC) solution of 1M LiF and 1M 12-crown-4 PC solution was used as the electrolyte in another battery. This crown ether acts as a cation acceptor to dissolve LiF in PC. The battery pack was cycled between approximately 3.2V and 5.5V at a rate of 1 mV / s.
[0095] Figure 4 Illustrated during the charge / discharge cycle by containing LiPF 6 The current provided by the battery does not show clear oxidation or reduction peaks in the figure. The battery has a much higher charge capacity than discharge capacity, indicating significant irreversibility.
[0096] Figure 5 The current supplied by a LiF-containing battery during charge / discharge cycling is illustrated showing oxidation peaks at about 3.6V and 4.15V and reduction peaks at about 4V...
Embodiment 3
[0098] Embodiment 3: Containing the fluoride solution of dissociating agent
[0099] Table 2 provides a summary of the experimental conditions that may be employed in the preparation of fluoride solutions of the present invention from various fluoride salts, including NH 4 F, NaF, KF, MgF 2 and AlF 3 .
[0100] Table 2: Examples of Fluoride Solutions
[0101] PC or EC / DMC crown ether anion receptor
[0102] Statement on Incorporation by Reference and Variations
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com