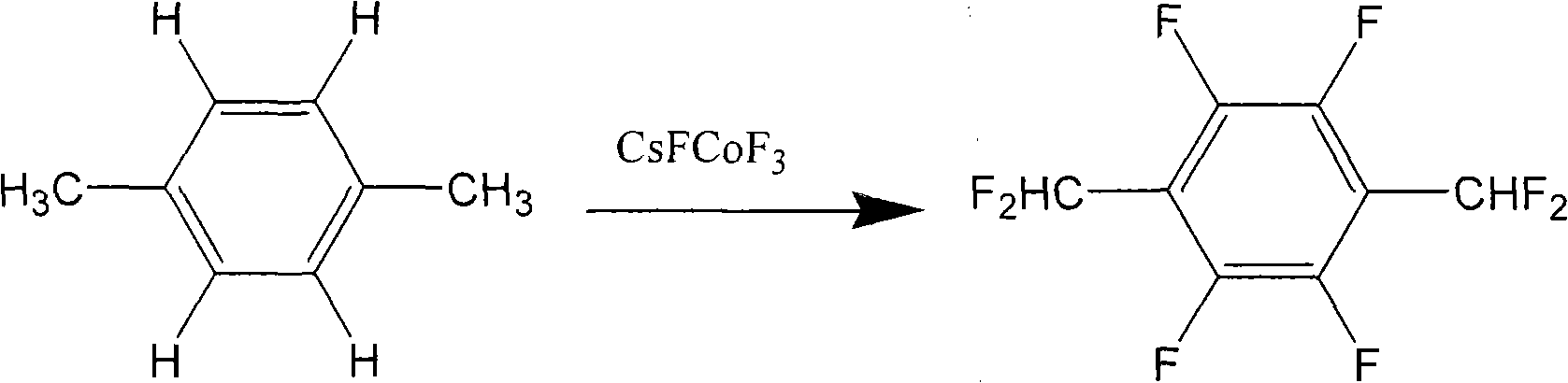
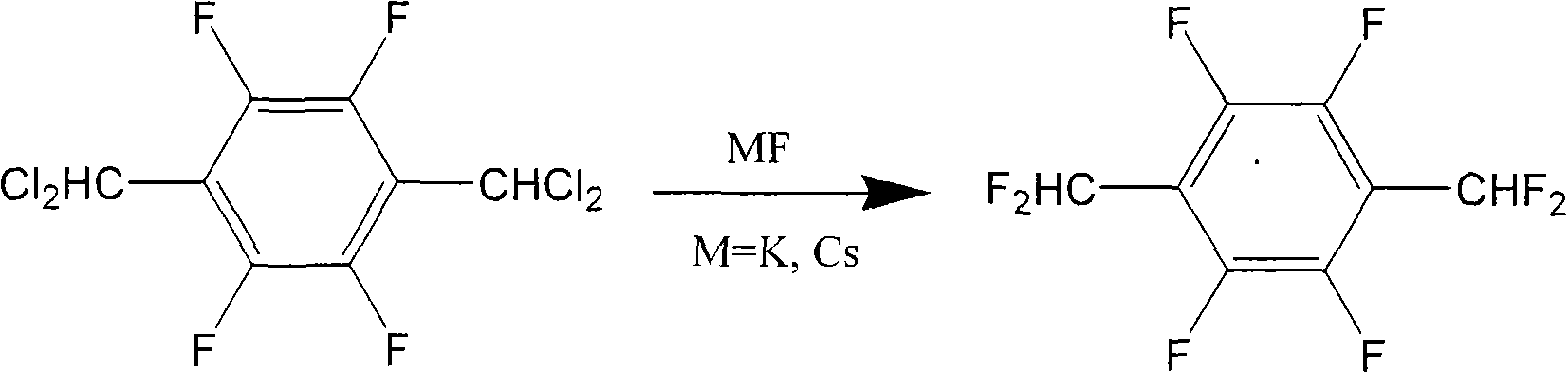
Method for preparing 1,4-bis(difluoromethyl) tetrafluorobenzene
A technology of difluoromethyl and tetrafluorobenzene, applied in the field of preparation 1, can solve the problems of many reaction by-products, difficulty, complicated fluorination reaction equipment and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]A condenser tube and magnetic stirring heating equipment were installed in the 150 ml reaction bottle. Then, 20 grams (0.063 moles) of 1,4-bis(dichloromethyl)tetrafluorobenzene (DCMTFB), 2 grams of 18-crown-6-ether (18-crown-6-ether) and 40 milliliters of acetonitrile ( Acetonitrile) was added in the reaction flask, and the stirring heater was turned on until the temperature of the oil bath was 80° C. and the above-mentioned organic reactants were completely dissolved. Next, 60 grams (0.39 moles) of fluorinating agent CsF were added, and the temperature was raised to 100° C. After reacting for 6 hours, the conversion rate of 1,4-bis(difluoromethyl)tetrafluorobenzene had reached more than 92%. The reaction crude product was treated by filtration, extraction, distillation and sublimation to obtain 12 grams of pure 1,4-bis(difluoromethyl)tetrafluorobenzene with a yield of 76%.
[0040] It is verified by product chemical analysis that the product obtained in this example is...
Embodiment 2
[0050] A condenser tube and magnetic stirring heating equipment were installed in the 150 ml reaction bottle. Then, 30 grams (0.095 moles) of 1,4-bis(dichloromethyl)tetrafluorobenzene (DCMTFB), 45 grams of KF, 45.8 grams of sulfolane (sulpholane) and 3 grams of tetraphenylphosphonium bromide were added to the reaction in the bottle. Turn on the stirring heater, and set the temperature of the oil bath at 125 to 130°C to react for 48 hours. In order to prevent the product from sublimating and overflowing, the outlet of the condenser tube can be plugged with a bottle stopper. Cool to room temperature after the reaction. Then, filter, filter cake with CH 2 Cl 2 After washing, the CH was distilled off under reduced pressure. 2 Cl 2 and 1,4-bis(difluoromethyl)tetrafluorobenzene (DFMTFB). Since 1,4-bis(difluoromethyl)tetrafluorobenzene has sublimation characteristics and accumulates on the condenser, therefore, CH 2 Cl 2 Cleaned, then remove CH 2 Cl 2 6.51 g of 1,4-bis(difl...
Embodiment 3 to 10
[0061] 5.00 grams (0.063 moles) of 1,4-bis(dichloromethyl)tetrafluorobenzene (DCMTFB), 0.50 grams of tetraphenylphosphine bromide (hereinafter referred to as PTC (Br)), 8.50 grams of KF and 30 grams of dimethyl Dimethylformamide (N, N-Dimethylformamide; DMF) was added into the reaction flask. Then, the mixture was heated to 120° C. in an oil bath and stirred with a magnetic stirrer. After 17 hours of reaction, a sample was taken for gas chromatography (GC) analysis, and 4F / 2F / DCMTFB=20.7 / 47.2 / 22.1 (GC area%) was obtained. Among them, 4F (following formula (1)), 3F (following formula (2)), 2F (following formula (3)), 1F (following formula (4)) and DCMTFB (following formula (5)) are respectively as follows ( 1) to (5):
[0062]
[0063] Formula (1) Formula (2)
[0064]
[0065] Formula (3) Formula (4)
[0066]
[0067] Formula (5)
[0068] The preparation method of Examples 4 to 10 is the same as that of Example 3. The relevant reactant dosages, experim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com